It is observed that Ni, with an atomic mass of 65.9291 u, decays by emission. Identify the nucleus that results from this decay 28- O 66 Co Cu 65 Co 25 Cu Submit Part B Request Answer If the nucleus found in part A has an atomic mass of 65.9289 u, what is the maximum kinetic energy of the emitted electron? Express your answer using one significant figure.
It is observed that Ni, with an atomic mass of 65.9291 u, decays by emission. Identify the nucleus that results from this decay 28- O 66 Co Cu 65 Co 25 Cu Submit Part B Request Answer If the nucleus found in part A has an atomic mass of 65.9289 u, what is the maximum kinetic energy of the emitted electron? Express your answer using one significant figure.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter21: Nuclear Chemistry
Section: Chapter Questions
Problem 43E: A B58 atom (mass = 8.0246 amu) decays into B48 atom (mass = 8.0053 amu) by loss of a + panrticle...
Related questions
Question
Find the radius of the hydrogen-atom Bohr orbit.
Answer A and B in first image.
Thank you

Transcribed Image Text:66
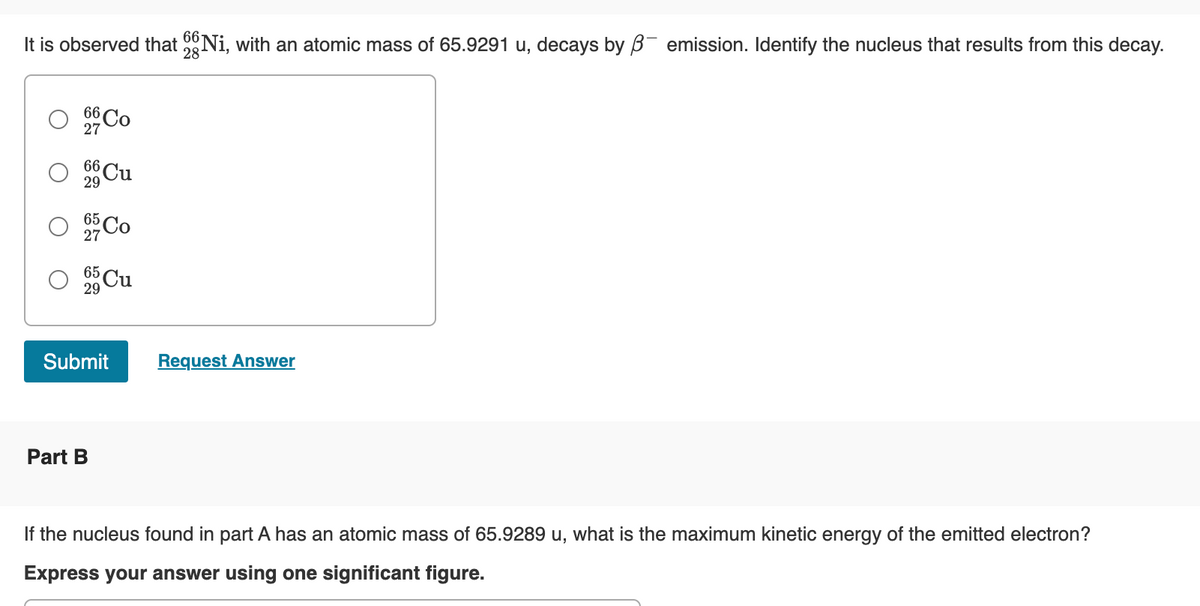
It is observed that Ni, with an atomic mass of 65.9291 u, decays by ß- emission. Identify the nucleus that results from this decay.
28
66 Co
27
29
O
66 Cu
O 95 Co
O 5 Cu
O
Submit
Part B
Request Answer
If the nucleus found in part A has an atomic mass of 65.9289 u, what is the maximum kinetic energy of the emitted electron?
Express your answer using one significant figure.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning