A fuel tank holds 22.3 gallons of gasoline. If the density of gasoline is 0.8206 g/mL , what is the mass in kilograms of gasoline in a full tank?
A fuel tank holds 22.3 gallons of gasoline. If the density of gasoline is 0.8206 g/mL , what is the mass in kilograms of gasoline in a full tank?
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.47PAE: 1.47 A student weighs 10 quarters and finds that their total mass is 56.63 grams. What should she...
Related questions
Question
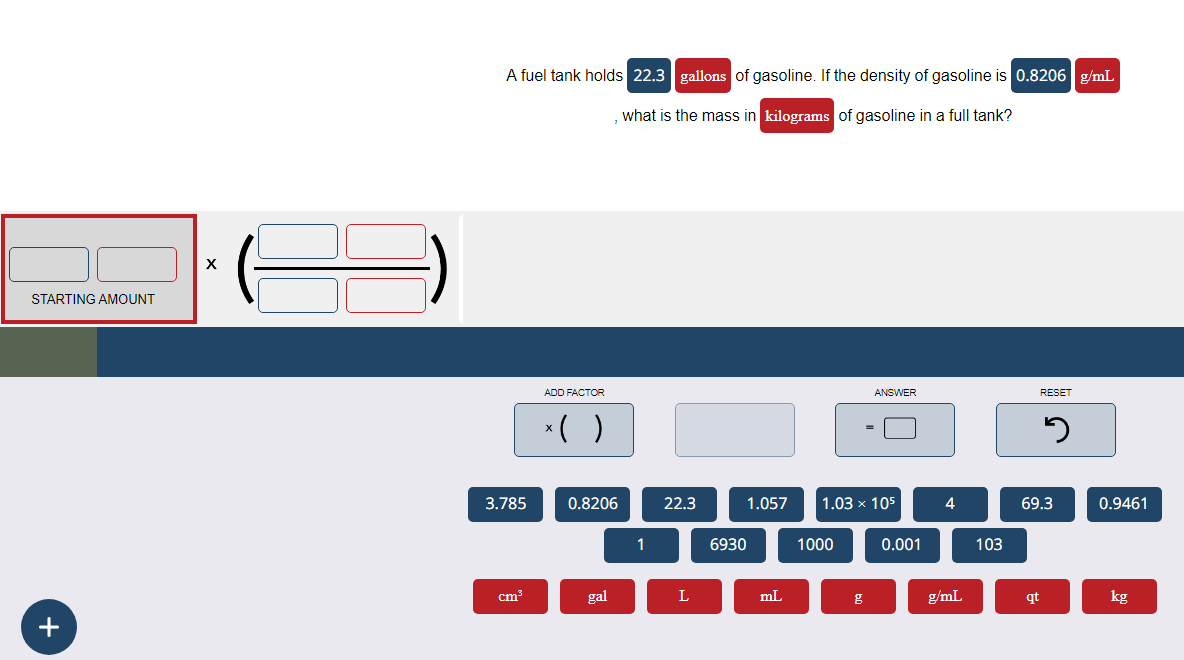
Transcribed Image Text:STARTING AMOUNT
+
X
A fuel tank holds 22.3 gallons of gasoline. If the density of gasoline is 0.8206 g/mL
, what is the mass in kilograms of gasoline in a full tank?
3.785
cm³
ADD FACTOR
x( )
0.8206
gal
1
22.3
L
6930
1.057 1.03 × 105
mL
ANSWER
1000
0.001
4
g/mL
103
RESET
qt
J
69.3
0.9461
kg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Incorrect,
It seems that either your starting amount is incorrect or one of your conversion factors has an error. Your current conversion gives units of L while your final answer should have units of kg. First, check your starting amount and unit to see if you have chosen the appropriate tiles. You should also consider if you might be missing factors or have included factors that are unnecessary.
Solution
Follow-up Question
This what it just said to me. Incorrect, So what information goes in the boxes in the parentheses
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning