IV) Complete and balance the following equations: N1aC Na2SO3 HСГ a) (gas evolution) KOH H2SO4 b) (acid-base) Fe(NO3)3 Sr(OH)2 c) (precipitation) V Write the ionic and net ionic equation for equations b) and c) of exercise IV. b) Ionic: Net ionic: с) Ionic: Net ionic: 60 + +
IV) Complete and balance the following equations: N1aC Na2SO3 HСГ a) (gas evolution) KOH H2SO4 b) (acid-base) Fe(NO3)3 Sr(OH)2 c) (precipitation) V Write the ionic and net ionic equation for equations b) and c) of exercise IV. b) Ionic: Net ionic: с) Ionic: Net ionic: 60 + +
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter7: Reactions In Aqueous Solutions
Section: Chapter Questions
Problem 8CR: ummarize the simple solubility rules for ionic compounds. How do we use these rules in determining...
Related questions
Question
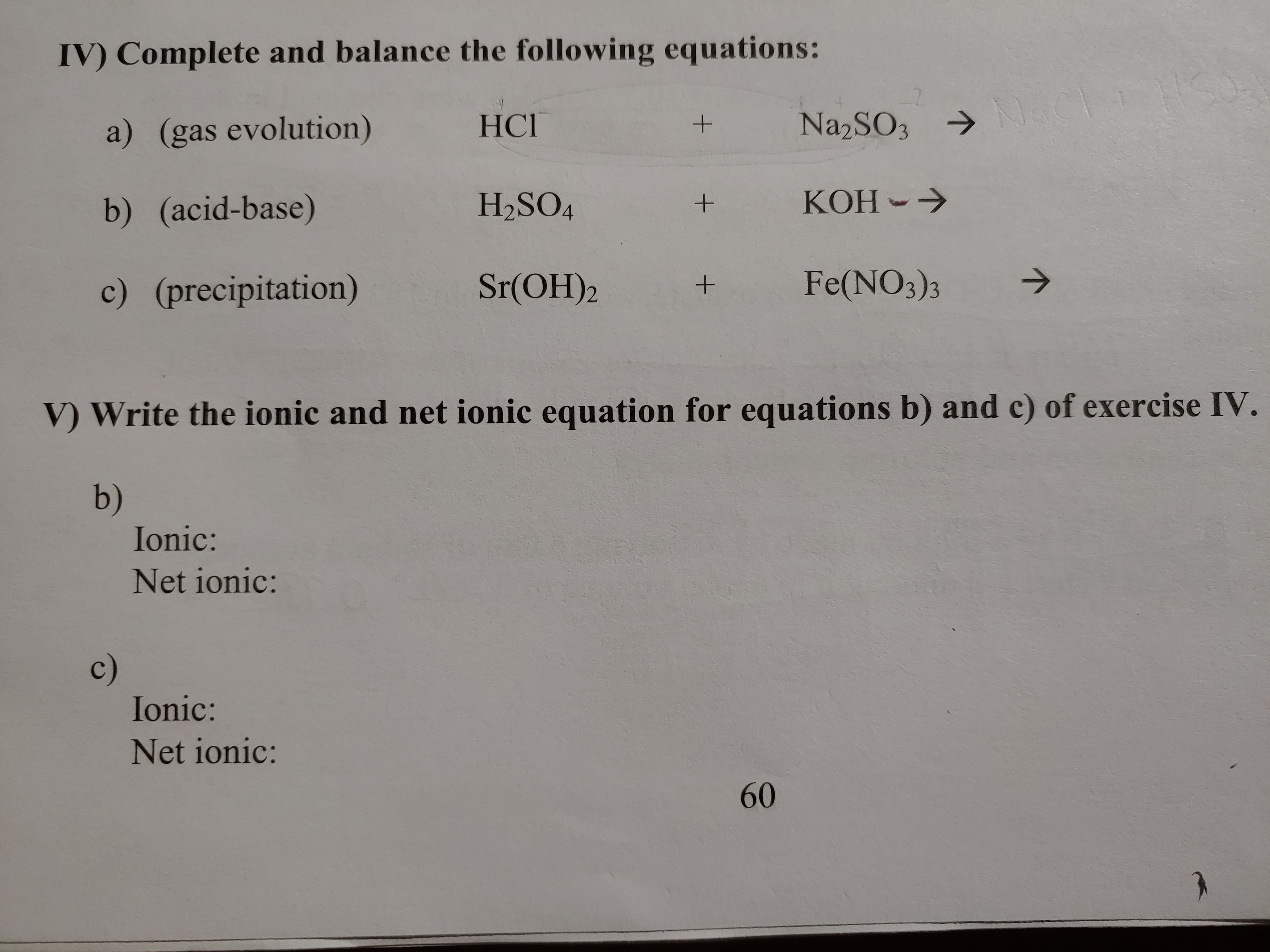
Transcribed Image Text:IV) Complete and balance the following equations:
N1aC
Na2SO3
HСГ
a) (gas evolution)
KOH
H2SO4
b) (acid-base)
Fe(NO3)3
Sr(OH)2
c) (precipitation)
V Write the ionic and net ionic equation for equations b) and c) of exercise IV.
b)
Ionic:
Net ionic:
с)
Ionic:
Net ionic:
60
+
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 6 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax