Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter8: Electrochemistry And Ionic Solutions
Section: Chapter Questions
Problem 8.47E: Determine the equilibrium constant for the reaction Sn+Pb2+Sn2++Pb
Related questions
Question
No need to solve anything under the calculations. I've provided it so that the, Explanation question could be answered.
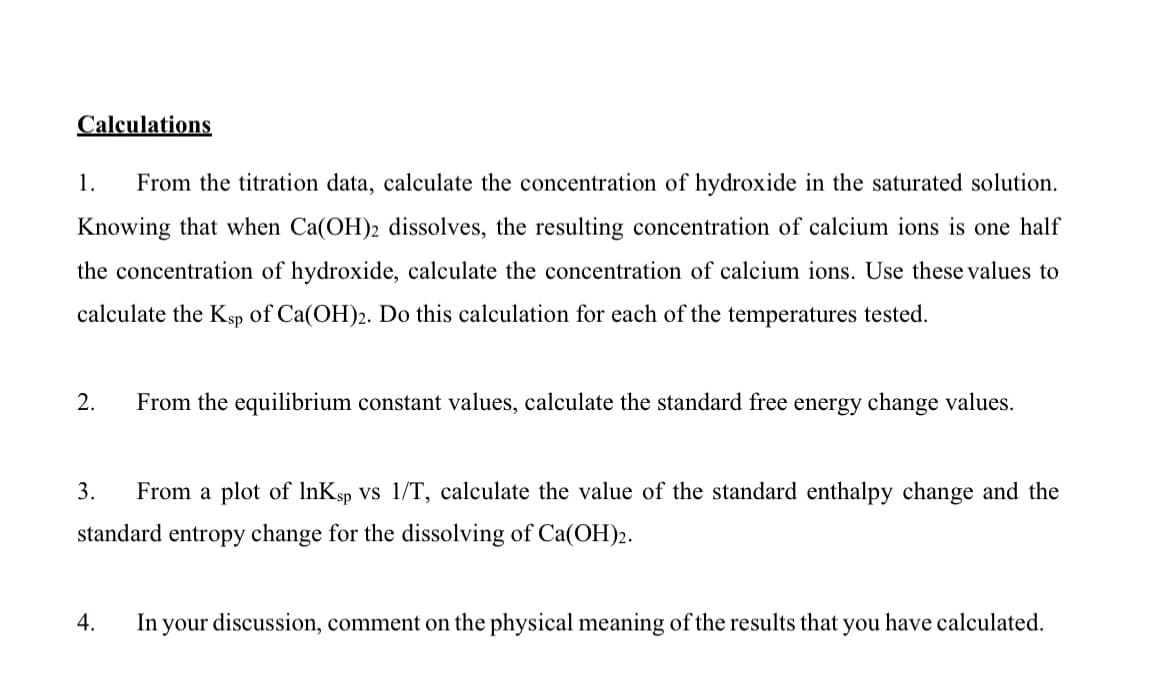
Transcribed Image Text:Calculations
1.
From the titration data, calculate the concentration of hydroxide in the saturated solution.
Knowing that when Ca(OH)2 dissolves, the resulting concentration of calcium ions is one half
the concentration of hydroxide, calculate the concentration of calcium ions. Use these values to
calculate the Ksp of Ca(OH)2. Do this calculation for each of the temperatures tested.
2.
From the equilibrium constant values, calculate the standard free energy change values.
3.
From a plot of InKsp Vs 1/T, calculate the value of the standard enthalpy change and the
standard entropy change for the dissolving of Ca(OH)2.
4.
In your discussion, comment on the physical meaning of the results that you have calculated.

Transcribed Image Text:2.
Explain how the following equation will be made use of in the calculations for this
experiment.
AG° = –RTlnK = AH° – TAS°
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,