Jeneen v The normal freezing point of a certain Iliquid X is -0.50 °C, but when 36.0 g of urea (CH,N,0) are dissolved in 800. g of X the solution freezes at -2.9 °C Instead. Use this information to calculate the molal freezing point depression constant K, of X. Be sure your answer is rounded to the correct number of significiant digits. do °C kg K; = 0 mol
Jeneen v The normal freezing point of a certain Iliquid X is -0.50 °C, but when 36.0 g of urea (CH,N,0) are dissolved in 800. g of X the solution freezes at -2.9 °C Instead. Use this information to calculate the molal freezing point depression constant K, of X. Be sure your answer is rounded to the correct number of significiant digits. do °C kg K; = 0 mol
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter6: An Introduction To Spectrometric Methods
Section: Chapter Questions
Problem 6.19QAP
Related questions
Question
Transcribed Image Text:O Collig Notes (1).pdf
A ALEKS - Jeneen Abdelra x
MMathway | Algebra Pro x G molar mass of zinc chl x
www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lix5uFZIVj2iEJjQd1WoxT77ErtzZpGbzESWhuQ5KnLFr-AdqJckJ3tzP4DtHEOm2H4pGXeoGQgUw9Q LCO06TW. *
Launch Meeting-Zoom x
G 0.16kg to g-Google Se x +
O GASES, LIQUIDS, AND SOLIDS
Using the Kf and Kb equations with electrolytes
Jeneen v
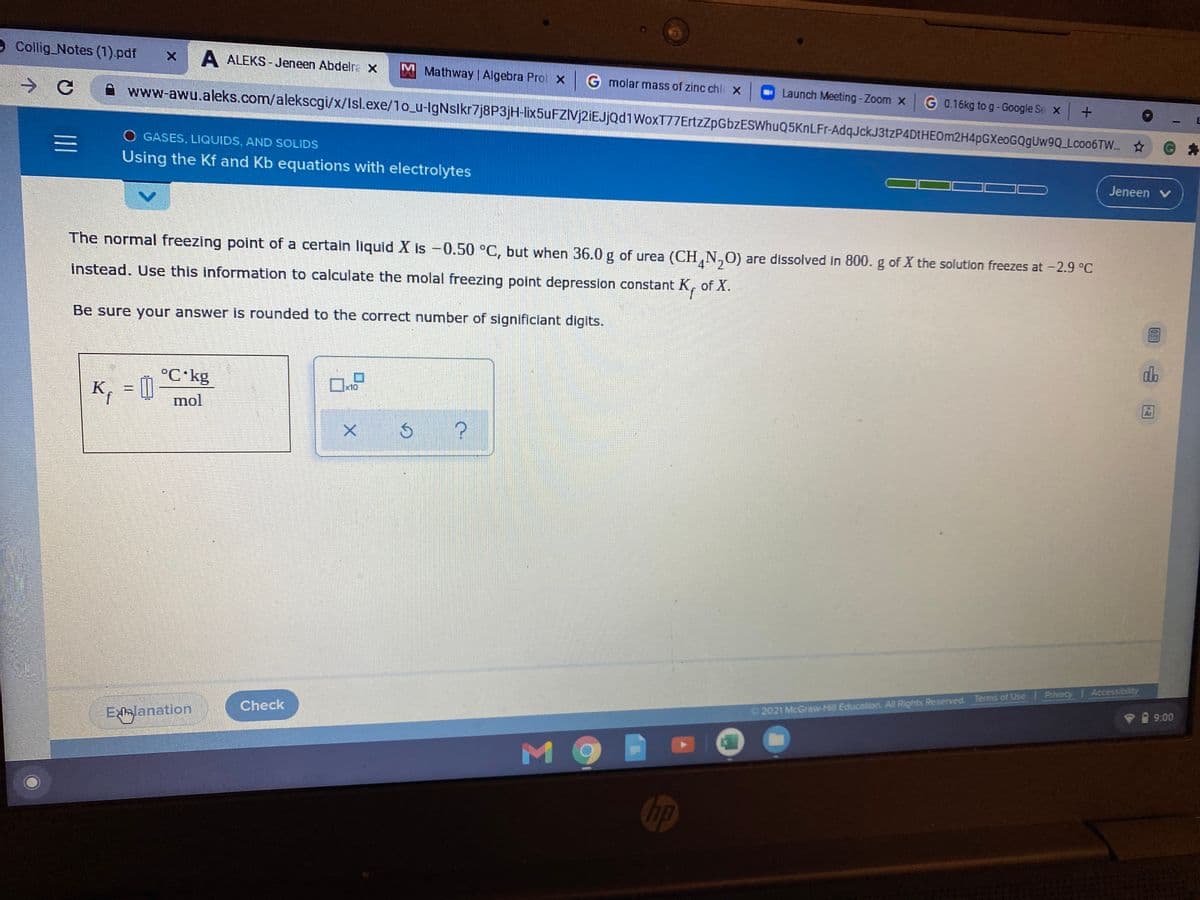
The normal freezing point of a certain liquid X is -0.50 °C, but when 36.0 g of urea (CH,N,O) are dissolved in 800. g of X the solution freezes at -2.9 °C
instead. Use this information to calculate the molal freezing point depression constant K, of X.
Be sure your answer is rounded to the correct number of significiant digits.
°C kg
K, = [|
x10
mol
Ar
Check
Exlanation
© 2021 McGraw-Hill Education All Rights Reserved. Terms of Use Privacy Accessibility
P 1 9:00
M 9
On
Expert Solution
Step 1

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning