Three fundamental particles of the atom are the m . At the center of each atom lies the atomic. ed consists of number of same number of and in the nucleus. e nucleus. All ato Isotope Hydrogen-1 Chlorine-36 Nitrogen-14 Potassium-40 Arsenic-75 Gold-197 thence, the same atomic number, us wi Isotopes are atoms that have the same number of number of is the total number of that has 6 and 6 the atomic mass number. A carbon isotope having 6 on the other hand, is carbon-14. 1. Complete the following table: which The atomic number refers to the f nu iw Jud. Electrons 1 All atoms of the same element have the 19 An isotope is identified by its atomic mass number, which and in the nucleus. A carbon isotope is identified as carbon-12, where 12 is and 8 Number of... Protons 550 17 33 Smorod cl qot mont gribas tirdog andlam sowol of eight mon 3. Which has more mass, a helium atom or a neon atom? un but todave to Neutrons Smuosted of got not guilbean adnion sniffe 4. Which has a greater number of atoms, a gram of helium or a gram of neon? 7 and 118 along books discla but a different SIGH gir or sawol most og at b Itroj 2. Which results in a more valuable product-adding or substracting protons from gold nuclel? ce la routW J I LIKE THE WAY YOUR ATOMS ARE PUT TOGETHER! N 8. or hisW+ •Subali-
Three fundamental particles of the atom are the m . At the center of each atom lies the atomic. ed consists of number of same number of and in the nucleus. e nucleus. All ato Isotope Hydrogen-1 Chlorine-36 Nitrogen-14 Potassium-40 Arsenic-75 Gold-197 thence, the same atomic number, us wi Isotopes are atoms that have the same number of number of is the total number of that has 6 and 6 the atomic mass number. A carbon isotope having 6 on the other hand, is carbon-14. 1. Complete the following table: which The atomic number refers to the f nu iw Jud. Electrons 1 All atoms of the same element have the 19 An isotope is identified by its atomic mass number, which and in the nucleus. A carbon isotope is identified as carbon-12, where 12 is and 8 Number of... Protons 550 17 33 Smorod cl qot mont gribas tirdog andlam sowol of eight mon 3. Which has more mass, a helium atom or a neon atom? un but todave to Neutrons Smuosted of got not guilbean adnion sniffe 4. Which has a greater number of atoms, a gram of helium or a gram of neon? 7 and 118 along books discla but a different SIGH gir or sawol most og at b Itroj 2. Which results in a more valuable product-adding or substracting protons from gold nuclel? ce la routW J I LIKE THE WAY YOUR ATOMS ARE PUT TOGETHER! N 8. or hisW+ •Subali-
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter3: Chemical Foundations: Elements, Atoms, And Ions
Section: Chapter Questions
Problem 60A
Related questions
Question
100%
pls send me ans of part 2 , 3 and 4 with explanation i will give you like sure.
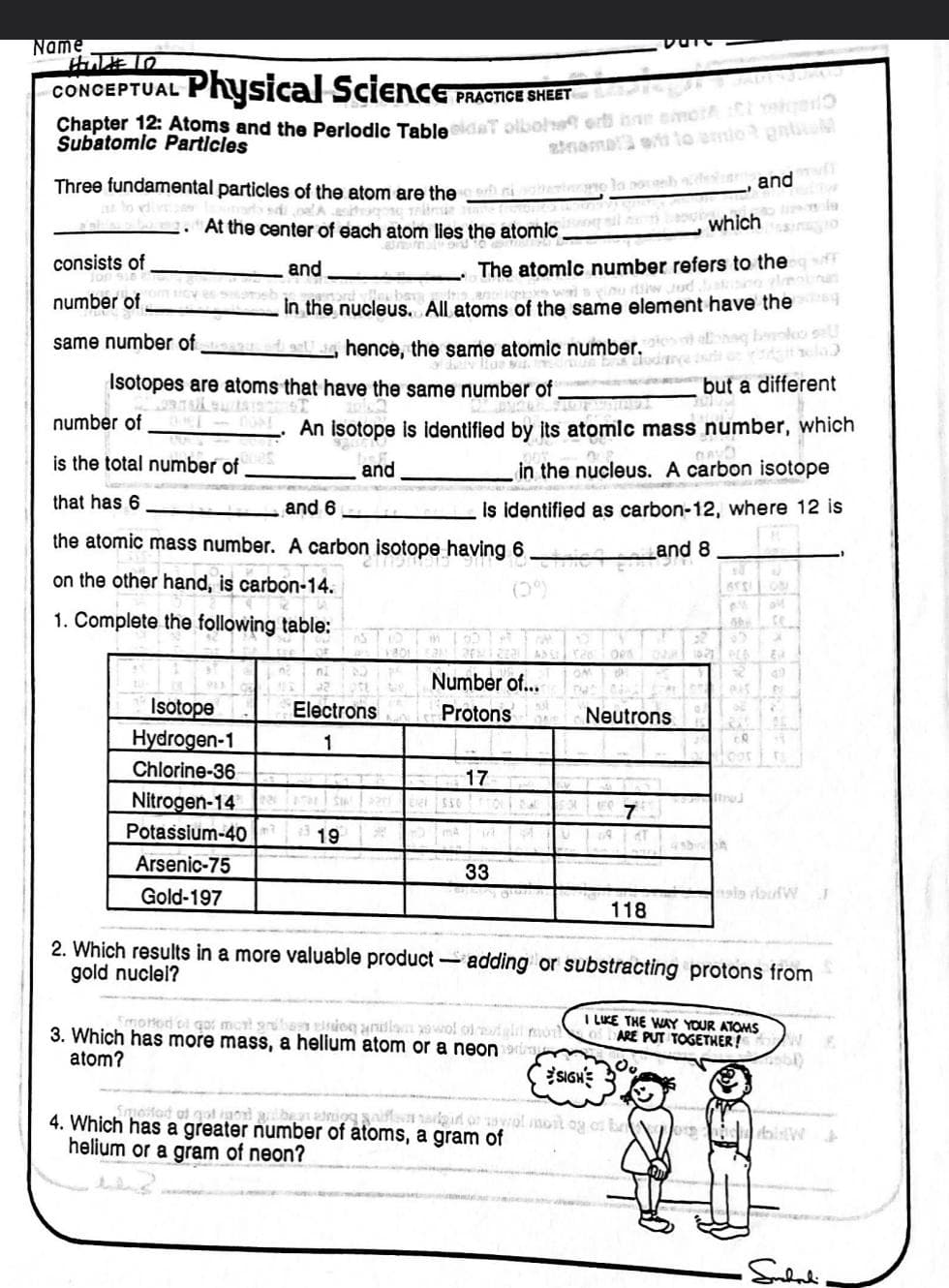
Transcribed Image Text:Name
HUL# 10
CONCEPTUAL
Physical Science PI
Chapter 12: Atoms and the Periodic Table eldaT olbohet er ne emoth C
Subatomic Particles
Three fundamental particles of the atom are the onesto la noceab sedeslut,
ar salAasthogong min
trimonies w
—a sitini allon. At the center of each atom lies the atomic
which
amalan?
The atomic number refers to the
the
others
in the nucleus. All atoms of the same element have the
PRACTICE SHEET
and
Isotope
Hydrogen-1
Chlorine-36
Isotopes are atoms that have the same number of
ORTA SYST
number of
is the total number of
that has 6
and 6
the atomic mass number. A carbon isotope having 6.
on the other hand, is carbon-14.
1. Complete the following table:
Nitrogen-14
Potassium-40
Arsenic-75
Gold-197
consists of
number of
d
same number of bedst, hence, the same atomic number of allonaq boynkou say
Imun bek lodrave isrit
but a different
An isotope is identified by its atomic mass number, which
and
in the nucleus. A carbon isotope
is identified as carbon-12, where 12 is
Electrons
1
19
sinema13 ei to amio? gnum
Number of...
Protons QAD
550
17
33
Smorted of got mest gribas nog andlowol of avigli mon
3. Which has more mass, a helium atom or a neon
atom?
Neutrons
7
SIGH
A
and 8.
118
10
2
BRAS
A
and
Itnej
aM
I LIKE THE WAY YOUR ATOMS
ARE PUT TOGETHER!
00
ce
X
EU
49
2. Which results in a more valuable product -adding or substracting protons from
gold nuclei?
d
la routW J
obl)
f
4. Which has a greater number of atoms, a gram of
Thested of not inert gal beat ad drog salfsen targard or swol most oy of boligbl
helium or a gram of neon?
•Suhaili,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning