JWhen 27.3 g KCJ Is added to a solution containing 28.3 g of Pb2+, a precipitate forms. The precipitate is filtered and drled and found to have a mass of 25.6 g. Determine the limiting reactant, theoretical yleld of PbCl2, and percent yleld for the reaction
JWhen 27.3 g KCJ Is added to a solution containing 28.3 g of Pb2+, a precipitate forms. The precipitate is filtered and drled and found to have a mass of 25.6 g. Determine the limiting reactant, theoretical yleld of PbCl2, and percent yleld for the reaction
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter19: The Chemistry Of The Main-group Elements
Section: Chapter Questions
Problem 81QRT: A natural brine found in Arkansas has a bromide ion concentration of 5.00 × 10−3 M. If 210. g Cl2...
Related questions
Question
Solve this bahin show evera stepo
10mins
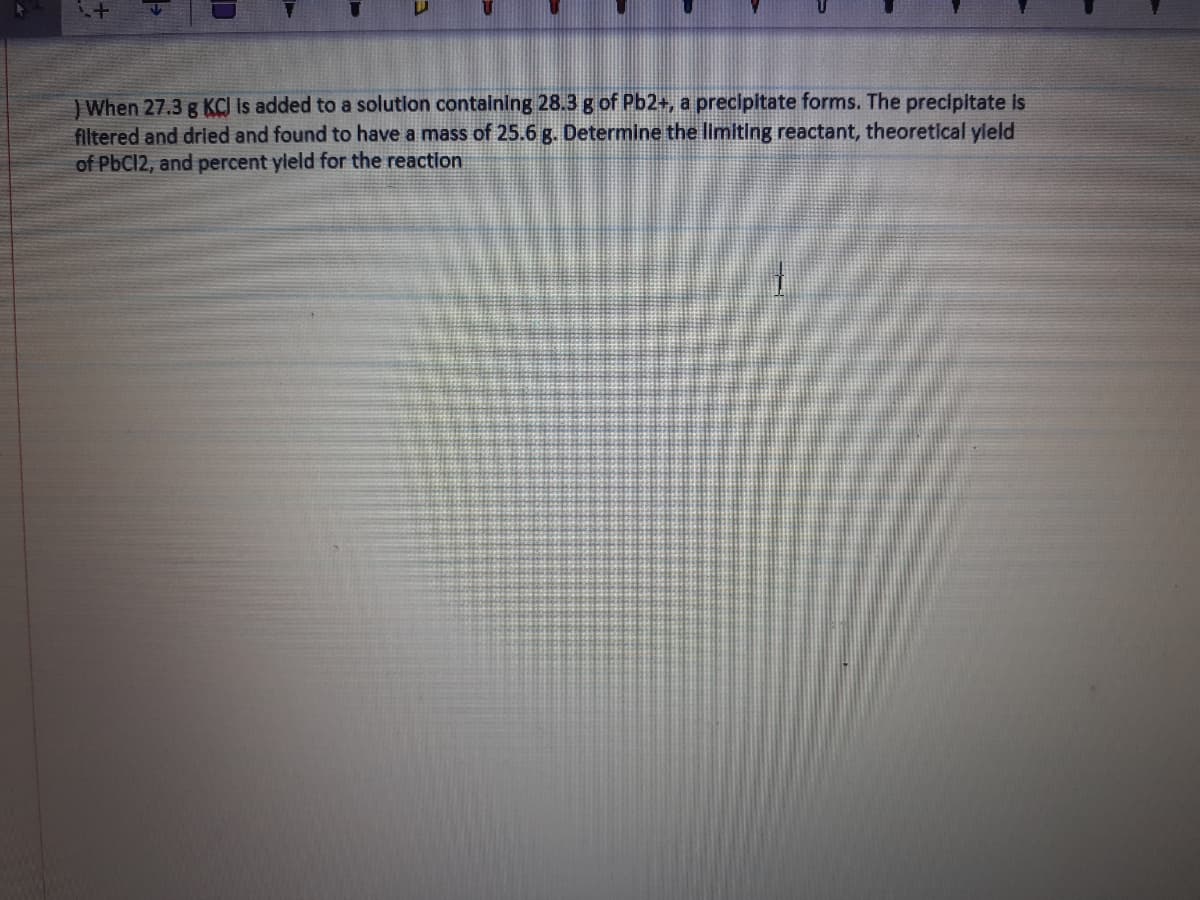
Transcribed Image Text:) When 27.3 g KCI Is added to a solution containing 28.3 g of Pb2+, a precipitate forms. The precipitate is
filtered and dried and found to have a mass of 25.6 g. Determine the limiting reactant, theoretical yleld
of PbC12, and percent yleld for the reaction
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning