Weight of Copper 1.006g Volume of Added Nitric Acid 5mL goy OVo stonr ar Total Weight of Added Sodium Carbonate 3.991g 57.118g 47736g Weight of Watch Glass and Filter Paper Weight of Watch Glass, Filter Paper and CuCl Precipitate 53.4849 Experimental Yield of CuCl 2,36616g Theoretical Yield of CuCl Percent Yield of CuCl Show your calculations for theoretical yield and percent yield below. Label each alculation. experimental yield=Damount cucl = (53.4849)-51.118 2.366g Theoretical yield of CuC w 1oggo ter aesggua notsvdo fW( cobbot mae s
Weight of Copper 1.006g Volume of Added Nitric Acid 5mL goy OVo stonr ar Total Weight of Added Sodium Carbonate 3.991g 57.118g 47736g Weight of Watch Glass and Filter Paper Weight of Watch Glass, Filter Paper and CuCl Precipitate 53.4849 Experimental Yield of CuCl 2,36616g Theoretical Yield of CuCl Percent Yield of CuCl Show your calculations for theoretical yield and percent yield below. Label each alculation. experimental yield=Damount cucl = (53.4849)-51.118 2.366g Theoretical yield of CuC w 1oggo ter aesggua notsvdo fW( cobbot mae s
Chapter20: Applications Of Oxidation/reduction Titrations
Section: Chapter Questions
Problem 20.26QAP
Related questions
Question
I need help on finding the theoretical yield
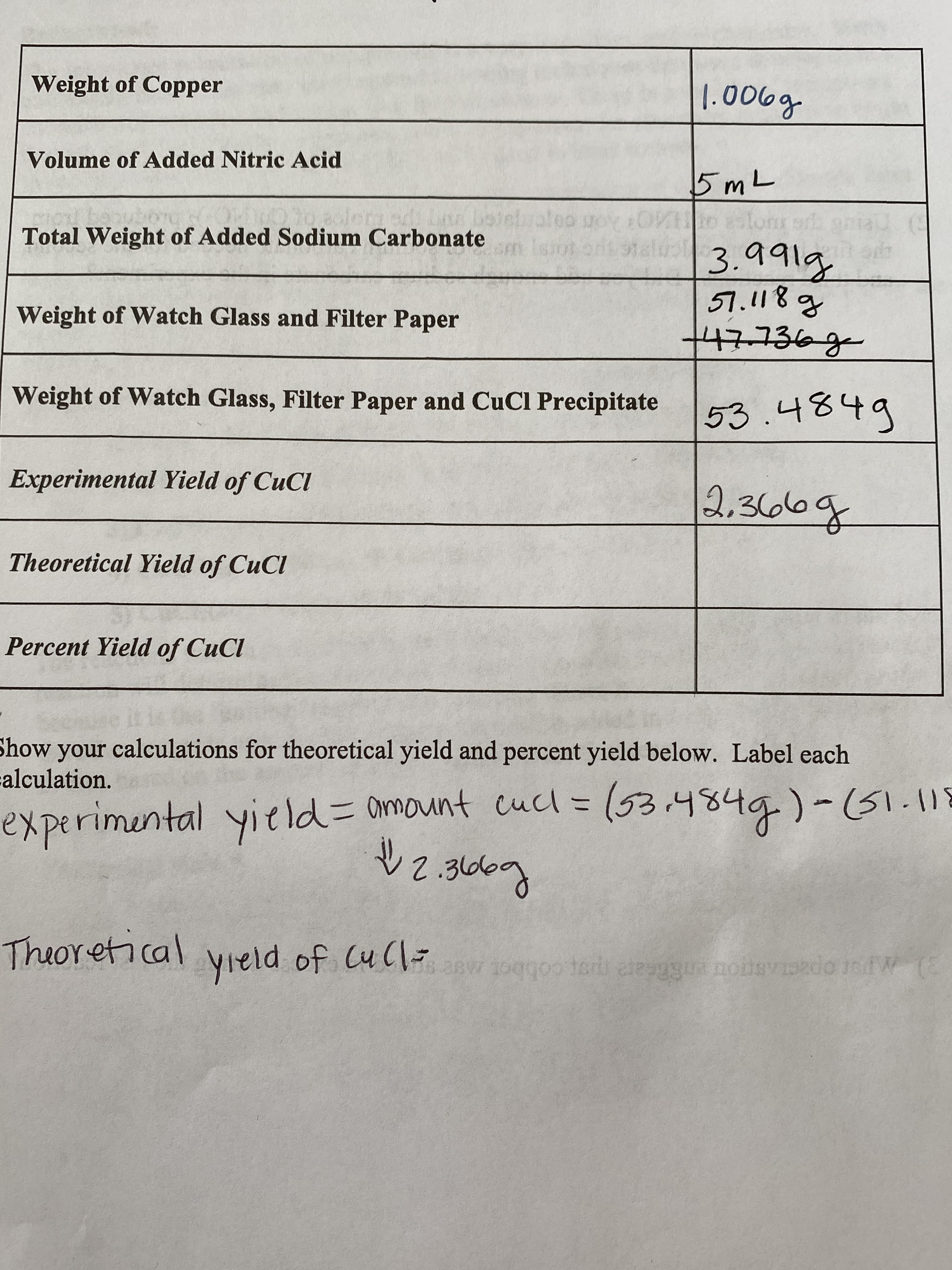
Transcribed Image Text:Weight of Copper
1.006g
Volume of Added Nitric Acid
5mL
goy OVo stonr ar
Total Weight of Added Sodium Carbonate
3.991g
57.118g
47736g
Weight of Watch Glass and Filter Paper
Weight of Watch Glass, Filter Paper and CuCl Precipitate 53.4849
Experimental Yield of CuCl
2,36616g
Theoretical Yield of CuCl
Percent Yield of CuCl
Show your calculations for theoretical yield and percent yield below. Label each
alculation.
experimental yield=Damount cucl = (53.4849)-51.118
2.366g
Theoretical
yield of CuC w 1oggo ter aesggua notsvdo fW(
cobbot mae s
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning