K2CO3(aq) + 2CUF(aq) → Cu2CO3(s) + 2KF(aq) The molecular equation you determined in Part B is shown above for your convenience. Examine each of the chemical species involved to determine the ions that would be present in solution. Be sure to consider both the coefficients and subscripts of the molecular equation, and then write this precipitation reaction in the form of a balanced complete ionic equation. Express your answer as a chemical equation including phases. Type an underscore (_) or a carat (^) to add subscripts and superscripts more quickly. • View Available Hint(s) ΑΣφ ? xª b +CO3 2+ →Cu,CO3(9) (aq) (aq) Submit Previous Answers
K2CO3(aq) + 2CUF(aq) → Cu2CO3(s) + 2KF(aq) The molecular equation you determined in Part B is shown above for your convenience. Examine each of the chemical species involved to determine the ions that would be present in solution. Be sure to consider both the coefficients and subscripts of the molecular equation, and then write this precipitation reaction in the form of a balanced complete ionic equation. Express your answer as a chemical equation including phases. Type an underscore (_) or a carat (^) to add subscripts and superscripts more quickly. • View Available Hint(s) ΑΣφ ? xª b +CO3 2+ →Cu,CO3(9) (aq) (aq) Submit Previous Answers
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 25QAP: Consider the following generic equation: H+(aq)+ B(aq)HB(aq)For which of the following pairs would...
Related questions
Question
100%
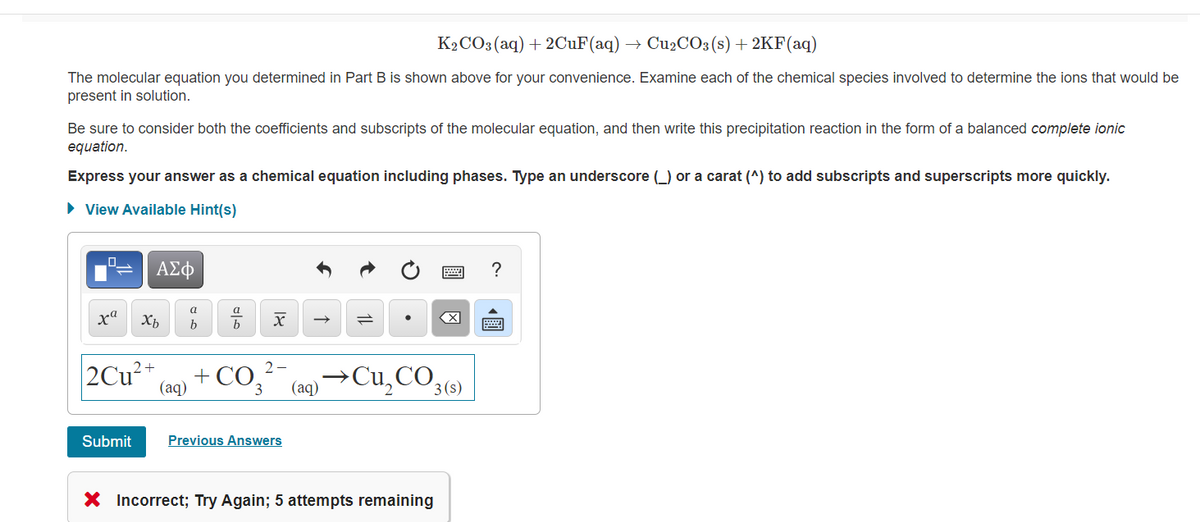
Transcribed Image Text:K2CO3(aq) + 2CUF(aq) → CU2CO3(s) + 2KF(aq)
The molecular equation you determined in Part B is shown above for your convenience. Examine each of the chemical species involved to determine the ions that would be
present in solution.
Be sure to consider both the coefficients and subscripts of the molecular equation, and then write this precipitation reaction in the form of a balanced complete ionic
equation.
Express your answer as a chemical equation including phases. Type an underscore (_) or a carat (^) to add subscripts and superscripts more quickly.
• View Available Hint(s)
?
ха
X
b
2Cu²*
+ CO3
2-
(aq) →Cu,CO3()
(aq)
Submit
Previous Answers
X Incorrect; Try Again; 5 attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning