30. A student is given 50.0 mL of a solution of Na,CO; of unknown concentration. To determine the concentration of the solution, the student mixes the solution with excess 1.0 M Ca(NO:),(ag), causing a precipitate to form. The balanced equation for the reaction is shown below. NazCOs(ag) + Ca(NO:)2(aq) --------> 2 NaNO:(aq) + CaCO:(s) a) Write the net ionic equation for the reaction that occurs when the solutions of Na,CO; and Ca(NO:); are mixed. b) The diagram below is incomplete. Draw in the species needed to accurately represent the major ionic species remaining in the solution after the reaction has been completed. (Na+ (NO, (NO, (Na (NO, (NO, Solid CaCO3
30. A student is given 50.0 mL of a solution of Na,CO; of unknown concentration. To determine the concentration of the solution, the student mixes the solution with excess 1.0 M Ca(NO:),(ag), causing a precipitate to form. The balanced equation for the reaction is shown below. NazCOs(ag) + Ca(NO:)2(aq) --------> 2 NaNO:(aq) + CaCO:(s) a) Write the net ionic equation for the reaction that occurs when the solutions of Na,CO; and Ca(NO:); are mixed. b) The diagram below is incomplete. Draw in the species needed to accurately represent the major ionic species remaining in the solution after the reaction has been completed. (Na+ (NO, (NO, (Na (NO, (NO, Solid CaCO3
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter4: Types Of Chemical Reactions And Solution Stoichiometry
Section: Chapter Questions
Problem 154CP: Triiodide ions are generated in solution by the following (unbalanced) reaction in acidic solution:...
Related questions
Question
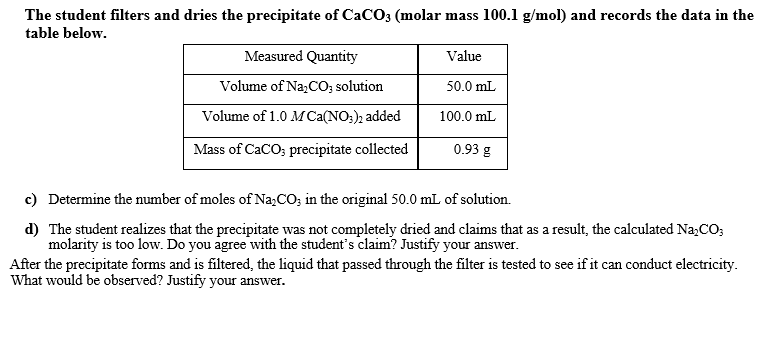
Transcribed Image Text:The student filters and dries the precipitate of CaCO3 (molar mass 100.1 g/mol) and records the data in the
table below.
Measured Quantity
Value
Volume of Na,CO; solution
50.0 mL
Volume of 1.0 M Ca(NO;), added
100.0 mL
Mass of CaCO; precipitate collected
0.93 g
c) Determine the number of moles of Na;CO; in the original 50.0 mL of solution.
d) The student realizes that the precipitate was not completely dried and claims that as a result, the calculated Na,CO;
molarity is too low. Do you agree with the student's claim? Justify your answer.
After the precipitate forms and is filtered, the liquid that passed through the filter is tested to see if it can conduct electricity.
What would be observed? Justify your answer.
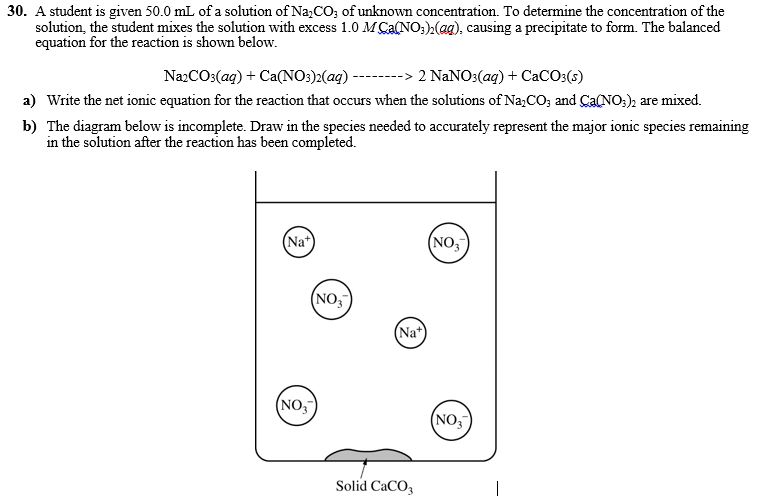
Transcribed Image Text:30. A student is given 50.0 mL of a solution of Na,CO; of unknown concentration. To determine the concentration of the
solution, the student mixes the solution with excess 1.0 M Ca(NO:),(ag), causing a precipitate to form. The balanced
equation for the reaction is shown below.
NazCO3(ag) + Ca(NO:)2(aq) --------> 2 NANO3(ag) + CaCO:(s)
a) Write the net ionic equation for the reaction that occurs when the solutions of Na,CO; and Ca(NO;), are mixed.
b) The diagram below is incomplete. Draw in the species needed to accurately represent the major ionic species remaining
in the solution after the reaction has been completed.
(Na*)
(NO,
(NO,
(Na*
(NO,
(NO,
Solid CaCO3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning