Klein, Organic Chemistry, 3e (a) QH H Locate each of these chiral centers: OH H Identify the configuration of each chiral center (for a position that is hot a chiral center, select "na) OH H 2 (b) Br Br Locate each of these chiral centers: 5 Br p lype here to search e DUNAUDIO P1 wwwt Klein, Organic Chemistry, 3e (C) OH Locate each of these chiral centers: 5 3 QH N 1 2 3 Identiry the configuration of each chhiral center (Tor a position that is not a chiral cernter, select "na"): 1 1 3 (d) HO of these chiral centers: Type here to search e r DUNAUDIO
Klein, Organic Chemistry, 3e (a) QH H Locate each of these chiral centers: OH H Identify the configuration of each chiral center (for a position that is hot a chiral center, select "na) OH H 2 (b) Br Br Locate each of these chiral centers: 5 Br p lype here to search e DUNAUDIO P1 wwwt Klein, Organic Chemistry, 3e (C) OH Locate each of these chiral centers: 5 3 QH N 1 2 3 Identiry the configuration of each chhiral center (Tor a position that is not a chiral cernter, select "na"): 1 1 3 (d) HO of these chiral centers: Type here to search e r DUNAUDIO
Chapter5: Stereochemistry At Tetrahedral Centers
Section5.2: The Reason For Handedness In Molecules: Chirality
Problem 3P
Related questions
Question
a,b,c,d
Transcribed Image Text:Klein, Organic Chemistry, 3e
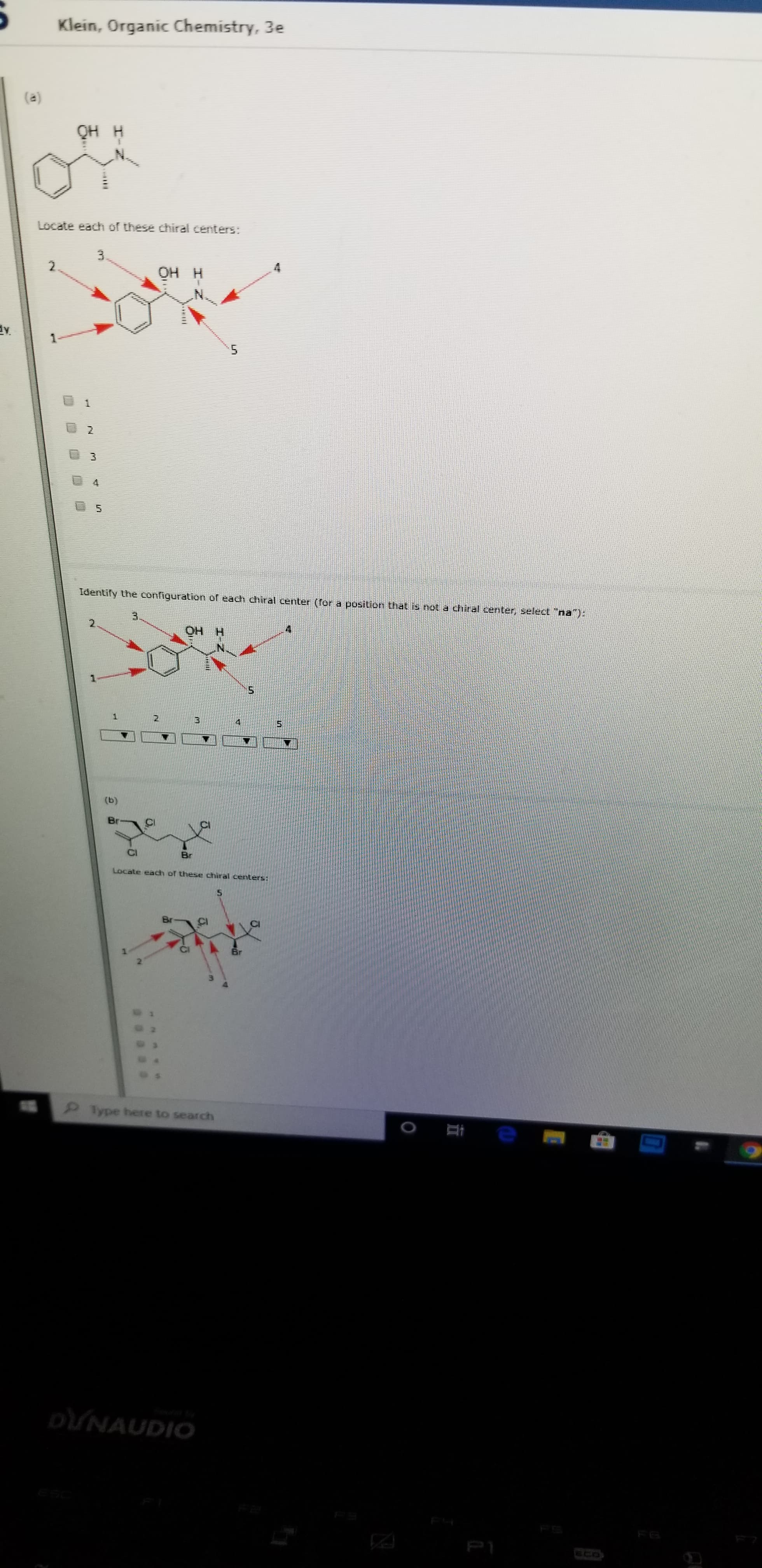
(a)
QH H
Locate each of these chiral centers:
OH H
Identify the configuration of each chiral center (for a position that is hot a chiral center, select "na)
OH H
2
(b)
Br
Br
Locate each of these chiral centers:
5
Br
p lype here to search
e
DUNAUDIO
P1
wwwt
Transcribed Image Text:Klein, Organic Chemistry, 3e
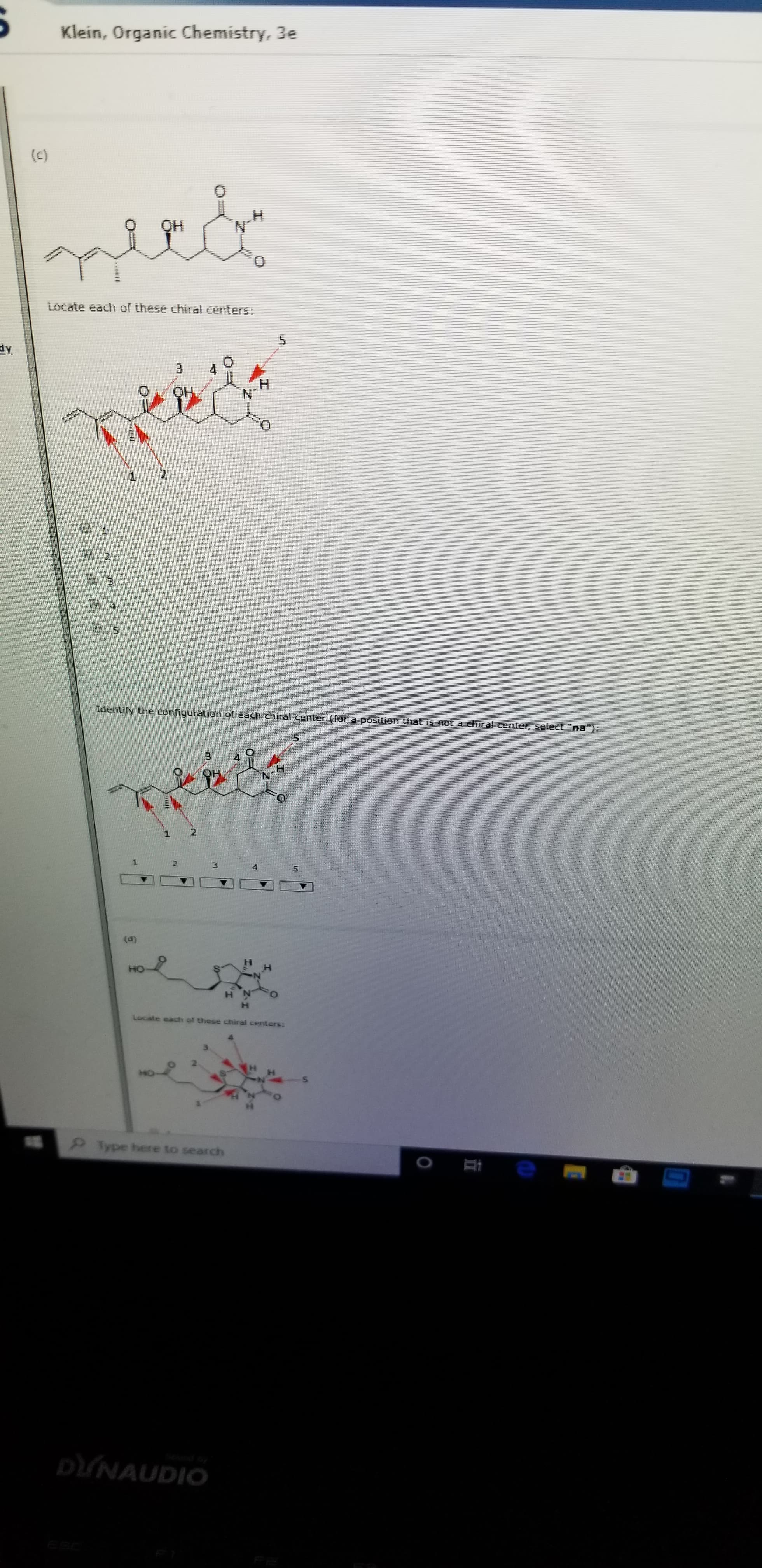
(C)
OH
Locate each of these chiral centers:
5
3
QH
N
1
2
3
Identiry the configuration of each chhiral center (Tor a position that is not a chiral cernter, select "na"):
1
1
3
(d)
HO
of these chiral centers:
Type here to search
e r
DUNAUDIO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

