KNO, 240 200 6160 NaCIO 120 KBr 80 KCI 40 Naci 20 40 60 80 100 120 Temperature °C If NaCIO3 has 100g of solute dissolved at 90°C, how much more solute can be added to make a saturated solution? O 40 O 80 O 100 O 180 Cop Solubility/100g of water
KNO, 240 200 6160 NaCIO 120 KBr 80 KCI 40 Naci 20 40 60 80 100 120 Temperature °C If NaCIO3 has 100g of solute dissolved at 90°C, how much more solute can be added to make a saturated solution? O 40 O 80 O 100 O 180 Cop Solubility/100g of water
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter8: Solutions
Section: Chapter Questions
Problem 8.9EP: A solution is made by dissolving 34.0 g of NaCl in 100 g of H2O at 0C. Based on the data in Table...
Related questions
Question
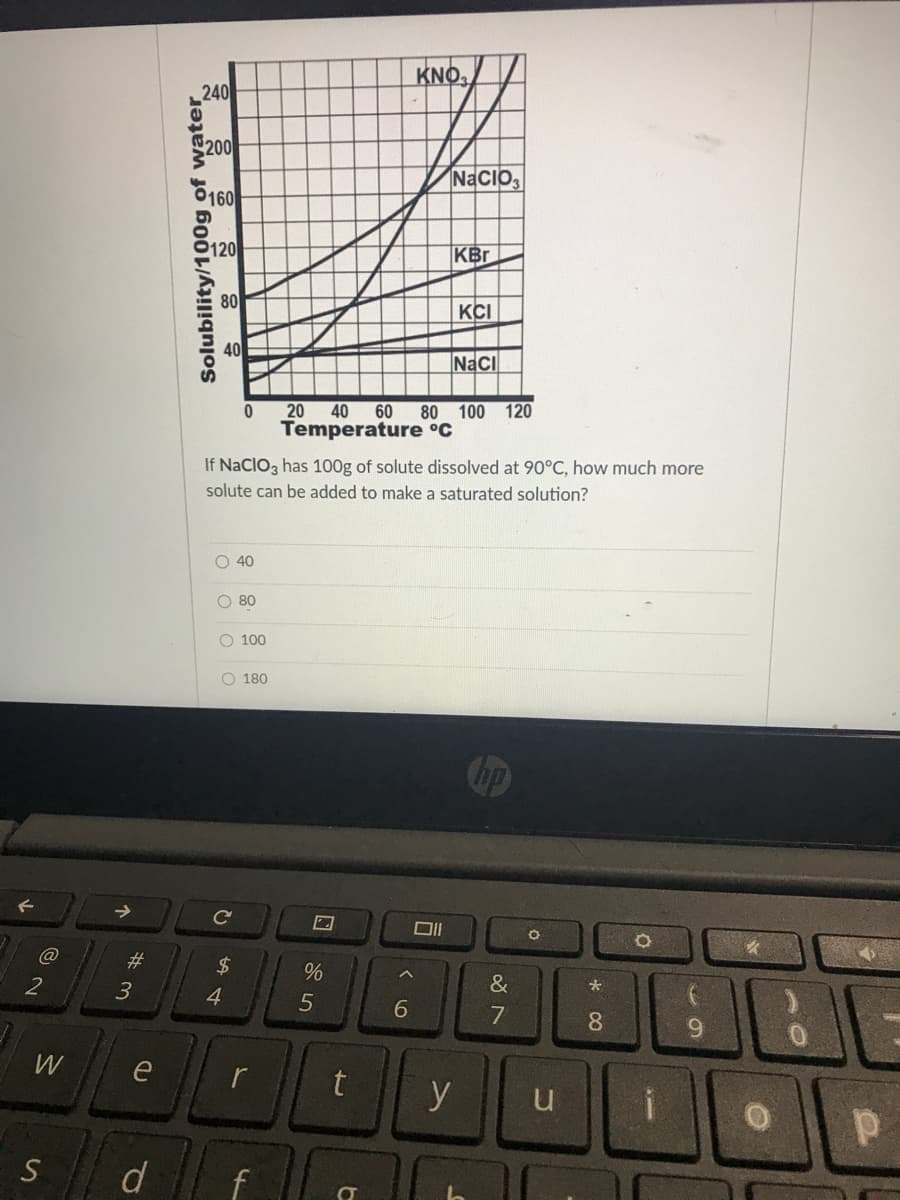
Transcribed Image Text:KNO,
240
200
NaCIO,
6160
P120
KBr
80
KCI
Naci
20 40
60
80 100 120
Temperature °C
If NaCIO3 has 100g of solute dissolved at 90°C, how much more
solute can be added to make a saturated solution?
O 40
O 80
O 100
O 180
Cip
->
Ce
%23
24
&
2
4
7
8
6.
W
e
y
d
f
Ss
o o oO
Solubility/100g of water
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning