Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 53QAP: The molarity of iodine in solution can be determined by titration with arsenious acid, H3AsO4. The...
Related questions
Question
Solve both parts otherwise I will downvote
![University Science Books
presented by Macmillan Learning
M
M
Rock, And Gallaghy de General Chemistry Publisher Univery Science
BMPM
General Chemistry 4th Edition
McQuarrie Rock Gallogly
K,SO, is a strong electrolyte.
Determine the concentration of each of the individual ions in a 0.750 M K. SO, solution.
[K¹] =
[SO] =](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F39b38033-afe0-42c2-8c00-6cc98e909b15%2F3584a323-5439-4dfa-a603-a4402c20d096%2Fbmxe4z_processed.jpeg&w=3840&q=75)
Transcribed Image Text:University Science Books
presented by Macmillan Learning
M
M
Rock, And Gallaghy de General Chemistry Publisher Univery Science
BMPM
General Chemistry 4th Edition
McQuarrie Rock Gallogly
K,SO, is a strong electrolyte.
Determine the concentration of each of the individual ions in a 0.750 M K. SO, solution.
[K¹] =
[SO] =
Transcribed Image Text:FUIT AS VIL
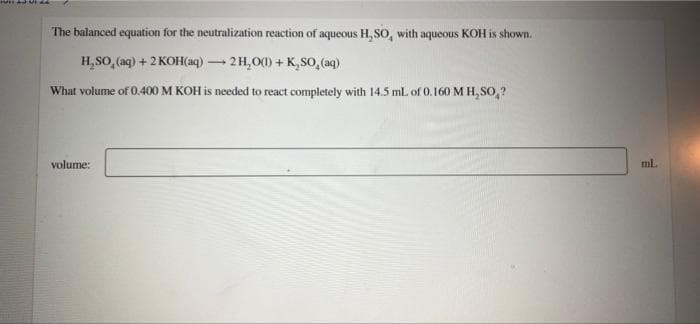
The balanced equation for the neutralization reaction of aqueous H, SO, with aqueous KOH is shown.
H₂SO₂ (aq) + 2KOH(aq) → 2H₂O(1) + K,SO, (aq)
What volume of 0.400 M KOH is needed to react completely with 14.5 mL of 0.160 M H₂SO₂?
volume:
ml.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning