Ksp OH" Cu2+ 4.8x10-20 2.3 Ni2+ 6.0x10-16 1.3 Zn2+ 3.0x10 16 1.0 O raise the pH to about 12 O bubble CO2 into the solution O lower the pH to about 2 O increase the sulfide level
Ksp OH" Cu2+ 4.8x10-20 2.3 Ni2+ 6.0x10-16 1.3 Zn2+ 3.0x10 16 1.0 O raise the pH to about 12 O bubble CO2 into the solution O lower the pH to about 2 O increase the sulfide level
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter13: The Chemistry Of Solutes And Solutions
Section: Chapter Questions
Problem 21QRT
Related questions
Question
I need the answer as soon as possible
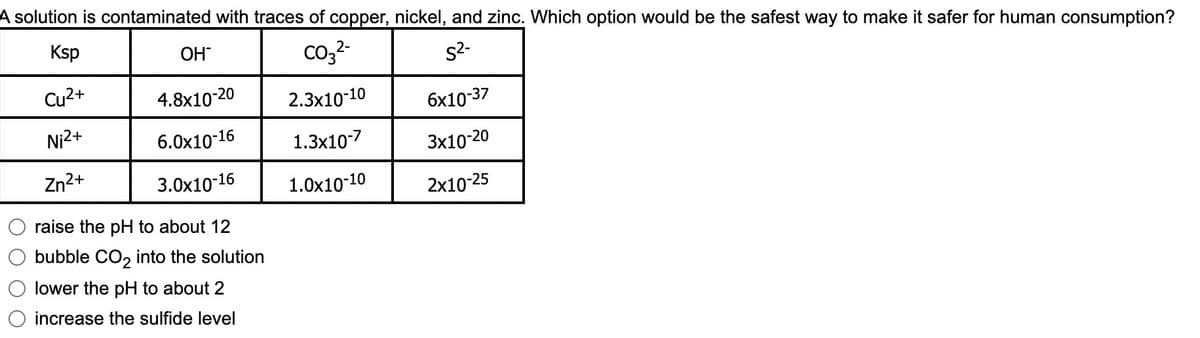
Transcribed Image Text:A solution is contaminated with traces of copper, nickel, and zinc. Which option would be the safest way to make it safer for human consumption?
Ksp
co,2-
OH
s2-
Cu2+
4.8x10-20
2.3x10-10
бх10-37
Ni2+
6.0x10-16
1.3x10-7
3х10-20
Zn2+
3.0x10-16
1.0x10-10
2x10-25
raise the pH to about 12
bubble CO, into the solution
lower the pH to about 2
increase the sulfide level
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning