L. Conductivity increases as ionic concentration increases II. Increasing temperature decreases metallic conductivity III. 1 Volt is the electrical potential that can deliver 1.6x10-19 J energy to 1 charge unit. IV. When 96485 current flow through an electrolysis cell, one equivalent gram of substance is released from the electrodes. Which or which of the above is wrong. Il ve IV A-) Ll ve ill B-) I ve il C-) Yalnız IV D-) Yalnız i E-) OOO O
L. Conductivity increases as ionic concentration increases II. Increasing temperature decreases metallic conductivity III. 1 Volt is the electrical potential that can deliver 1.6x10-19 J energy to 1 charge unit. IV. When 96485 current flow through an electrolysis cell, one equivalent gram of substance is released from the electrodes. Which or which of the above is wrong. Il ve IV A-) Ll ve ill B-) I ve il C-) Yalnız IV D-) Yalnız i E-) OOO O
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 43P
Related questions
Question
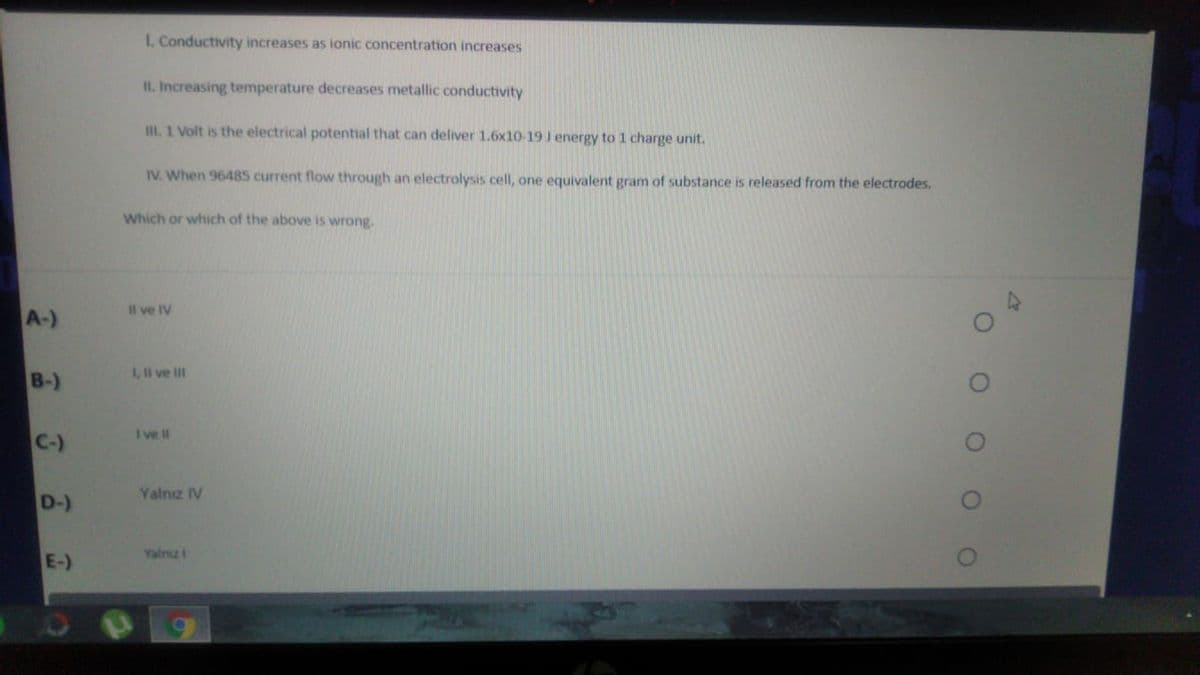
Transcribed Image Text:I. Conductivity increases as ionic concentration increases
II. Increasing temperature decreases metallic conductivity
III. 1 Volt is the electrical potential that can deliver 1.6x10-19 J energy to 1 charge unit.
IV. When 96485 current flow through an electrolysis cell, one equivalent gram of substance is released from the electrodes.
Which or which of the above is wrong.
Il ve IV
A-)
1, l ve II
B-)
I ve II
C-)
Yalnz IV
D-)
Yalnız i
E-)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning