Lab Data You have not titrated to the endpoint Initial volume of buret (mL) Mass of KHP (g) Observations turned purple Final volume of buret (mL) Volume of NaOH (mL) Concentration of NaOH (M) 1.90 1.300 How to calculate sodium hydroxide concentration ESET -29 -30 -31 -32 -33 MY NOTES A LAB DATA SHOW LABELS 1 Turn on stir plate PHASE 6: Titration 2 Start titration by adding sodium hydroxide to Erlenmeyer flask 4 Complete the following steps: 3 Record your observations in Lab Data 5 6 Stop titration t endpoint. Record final buret volume and volume of NaOH in Lab Data Turn off stir plate Calculate molarity of NaOH solution. Record concentration in Lab Data
Lab Data You have not titrated to the endpoint Initial volume of buret (mL) Mass of KHP (g) Observations turned purple Final volume of buret (mL) Volume of NaOH (mL) Concentration of NaOH (M) 1.90 1.300 How to calculate sodium hydroxide concentration ESET -29 -30 -31 -32 -33 MY NOTES A LAB DATA SHOW LABELS 1 Turn on stir plate PHASE 6: Titration 2 Start titration by adding sodium hydroxide to Erlenmeyer flask 4 Complete the following steps: 3 Record your observations in Lab Data 5 6 Stop titration t endpoint. Record final buret volume and volume of NaOH in Lab Data Turn off stir plate Calculate molarity of NaOH solution. Record concentration in Lab Data
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 51P
Related questions
Question
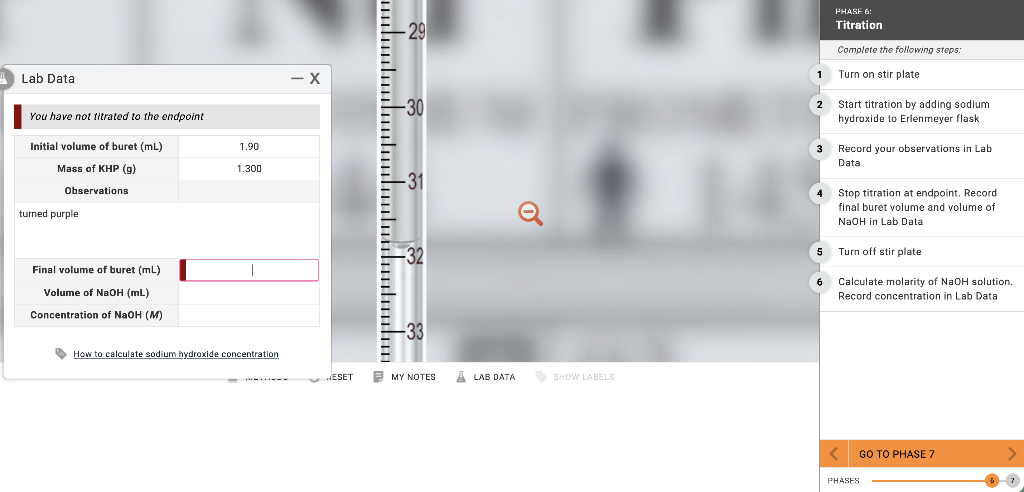
Transcribed Image Text:Lab Data
You have not titrated to the endpoint
Initial volume of buret (mL)
Mass of KHP (g)
Observations
turned purple
Final volume of buret (ml)
Volume of NaOH (mL)
Concentration of NaOH (M)
1.90
1.300
T
How to calculate sodium hydroxide concentration
- X
ESET
-29
-30
-31
-32
MY NOTES
ALAB DATA
SHOW LABELS
1
3
2 Start titration by adding sodium
hydroxide to Erlenmeyer flask
5
PHASE 6:
Titration
6
Complete the following steps:
Turn on stir plate
Record your observations in Lab
Data
Stop titration at endpoint. Record
final buret volume and volume of
NaOH in Lab Data
Turn off stir plate
Calculate molarity of NaOH solution.
Record concentration in Lab Data
PHASES
GO TO PHASE 7
>
7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning