Label the diagram below with the following terms: Solid, Liquid, Gas, Vaporization, Freezing, Melting Condensation, Cools Cools Cools Warms Warms Warms Heat Energy Temperature
Label the diagram below with the following terms: Solid, Liquid, Gas, Vaporization, Freezing, Melting Condensation, Cools Cools Cools Warms Warms Warms Heat Energy Temperature
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter15: Gases,liquids, And Solids
Section: Chapter Questions
Problem 61E
Related questions
Question
READ THE FOLLOWING AND PROVIDE WHAT IS ASKED MAKE SURE YOU DON'T MISS ANYTHING :)
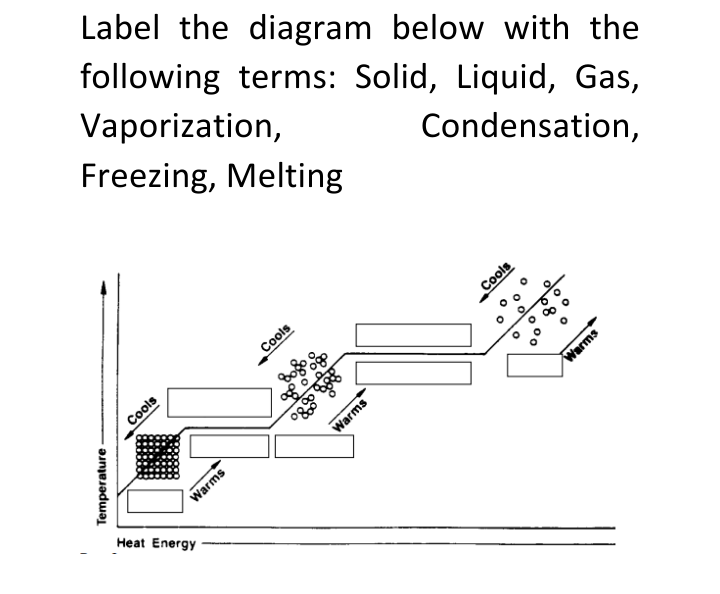
Transcribed Image Text:Label the diagram below with the
following terms: Solid, Liquid, Gas,
Vaporization,
Freezing, Melting
Condensation,
Cools
Cools
Cools
Warms
Warms
Warms
Heat Energy
Temperature
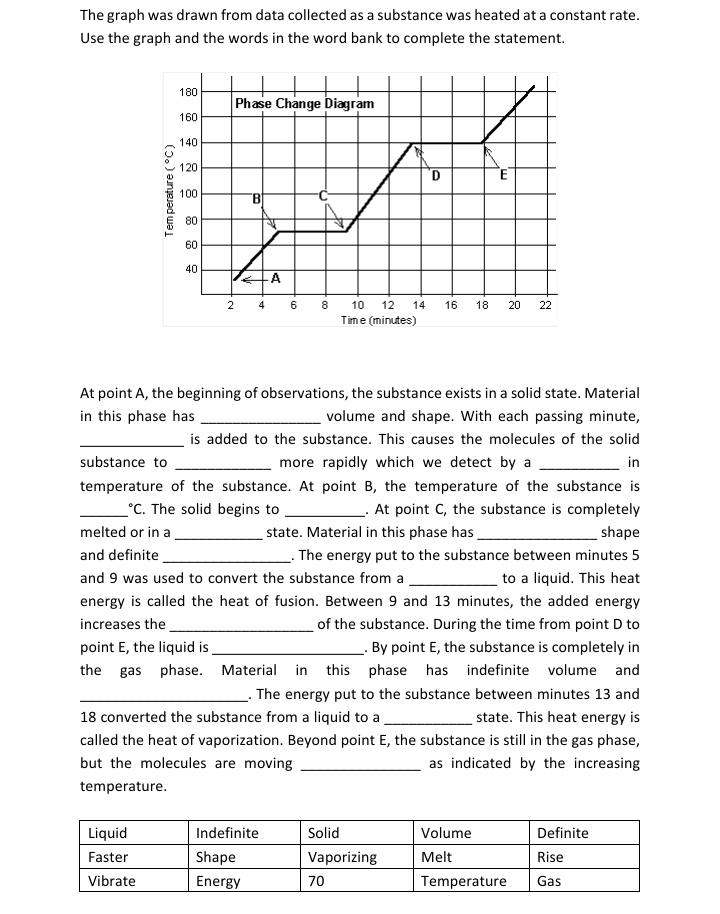
Transcribed Image Text:The graph was drawn from data collected as a substance was heated at a constant rate.
Use the graph and the words in the word bank to complete the statement.
180
Phase Change Diagram
160
140
120
D
100
80
60
40
A
8 10 12
Time (minutes)
2
4
6
14 16
18
20
22
At point A, the beginning of observations, the substance exists in a solid state. Material
in this phase has
volume and shape. With each passing minute,
is added to the substance. This causes the molecules of the solid
substance to
more rapidly which we detect by a
in
temperature of the substance. At point B, the temperature of the substance is
°C. The solid begins to
At point C, the substance is completely
state. Material in this phase has
. The energy put to the substance between minutes 5
melted or in a
shape
and definite
and 9 was used to convert the substance from a
to a liquid. This heat
energy is called the heat of fusion. Between 9 and 13 minutes, the added energy
increases the
point E, the liquid is.
of the substance. During the time from point D to
_- By point E, the substance is completely in
the gas phase. Material in this phase has indefinite volume and
The energy put to the substance between minutes 13 and
18 converted the substance from a liquid to a
state. This heat energy is
called the heat of vaporization. Beyond point E, the substance is still in the gas phase,
but the molecules are moving
as indicated by the increasing
temperature.
Liquid
Indefinite
Solid
Volume
Definite
Faster
Shape
Vaporizing
Melt
Rise
Vibrate
Energy
70
Temperature
Gas
Temperature (°C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning