lass, we learned about radical halogenation. Instead of using 12 or C12 to halogenate propane, ou can use iodine monochloride, I-CI. hv X= Cl or I + HCI When performing this reaction, it can be observed that one of the four possible products is ormed in the greatest yield. Using your chemical intuition and the bond dissociation energies rovided on separate sheet, determine which product is formed in the greatest yield. Use the pace below for work/calculations.
lass, we learned about radical halogenation. Instead of using 12 or C12 to halogenate propane, ou can use iodine monochloride, I-CI. hv X= Cl or I + HCI When performing this reaction, it can be observed that one of the four possible products is ormed in the greatest yield. Using your chemical intuition and the bond dissociation energies rovided on separate sheet, determine which product is formed in the greatest yield. Use the pace below for work/calculations.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter20: Acidity And Pka Of Phenols
Section: Chapter Questions
Problem 11E
Related questions
Question
Can someone please explain and show work / calculations? Please use an outside
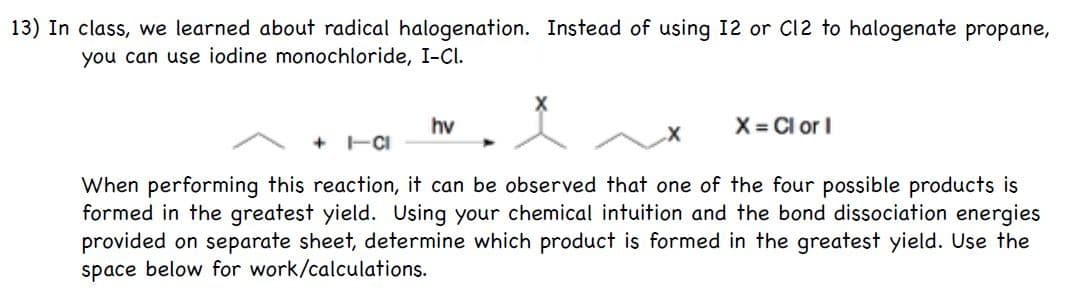
Transcribed Image Text:13) In class, we learned about radical halogenation. Instead of using 12 or C12 to halogenate propane,
you can use iodine monochloride, I-Cl.
hv
X = Cl or I
+ -CI
When performing this reaction, it can be observed that one of the four possible products is
formed in the greatest yield. Using your chemical intuition and the bond dissociation energies
provided on separate sheet, determine which product is formed in the greatest yield. Use the
space below for work/calculations.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning