LEFT silver (Ag) RIGHT copper (Cu) zinc VOLTAGE +0.43 11.50 MACROSCOPIC OBSERVATIONS Purple right-side solution and metal warn; left side metal has deposits. Right metal warns away: MICROSCOPIC OBSERVATIONS Left side electrons flow downward, attracting additional Ag atoms to join the surface, while right side electrons travel upward, ionizing and dispersing the metal atoms into the solution. Zinc releases two clectrons and passes into the solution as 2n+2 making the electrode negatively charged. Aga extracted on LEFT SIDE INFERENCES cathode, reduction, Ag 1 le--> Ago Reduction Cathode RIGHT SIDE INFERENCES anode, oxidation, CUO-> Cu+2+2e- Oxidation
LEFT silver (Ag) RIGHT copper (Cu) zinc VOLTAGE +0.43 11.50 MACROSCOPIC OBSERVATIONS Purple right-side solution and metal warn; left side metal has deposits. Right metal warns away: MICROSCOPIC OBSERVATIONS Left side electrons flow downward, attracting additional Ag atoms to join the surface, while right side electrons travel upward, ionizing and dispersing the metal atoms into the solution. Zinc releases two clectrons and passes into the solution as 2n+2 making the electrode negatively charged. Aga extracted on LEFT SIDE INFERENCES cathode, reduction, Ag 1 le--> Ago Reduction Cathode RIGHT SIDE INFERENCES anode, oxidation, CUO-> Cu+2+2e- Oxidation
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
100%
Be sure to follow all steps.
First, specifically describe the role of copper and then Zinc in the redox process in full.
Be sure to include half reactions for each metal and a description of macroscopic observations you may expect when testing the experimental design.
Example of macroscopic observations and half reactions are attached.
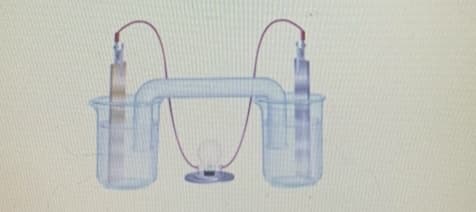
Transcribed Image Text:IN

Transcribed Image Text:LEFT RIGHT
silver
(Ag)
Iver
copper
(Cu)
zinc
VOLTAGE
+0.43
+1.53
MACROSCOPIC
OBSERVATIONS
Purple right-side solution and
metal warn; left side metal
has deposits.
Right metal warns away:
MICROSCOPIC
OBSERVATIONS
Left side electrons flow downward, attracting
additional Ag atoms to join the surface, while right
side electrons travel upward, ionizing and
dispersing the metal atoms into the solution.
Zinc releases two electrons and passes into the solution as 2n+2
making the electrode negatively charged Age extracted one
LEFT SIDE
INFERENCES
cathode, reduction,
Ag 1 le-> Ago
Reduction
Cathode
RIGHT SIDE
INFERENCES
anode, oxidation,
CUO-->
Cu 2+2e-
Oxidation
Expert Solution
Step 1
Redox reactions are reactions which involve the transfer of electrons from one species to another species. The species that loses electrons is said to be oxidized while the species that gains electrons is said to be reduced.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY