Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 100AP: f you have equal mole samples of NO2 and F2 , which of the following must be true? l type='a'> The...
Related questions
Question
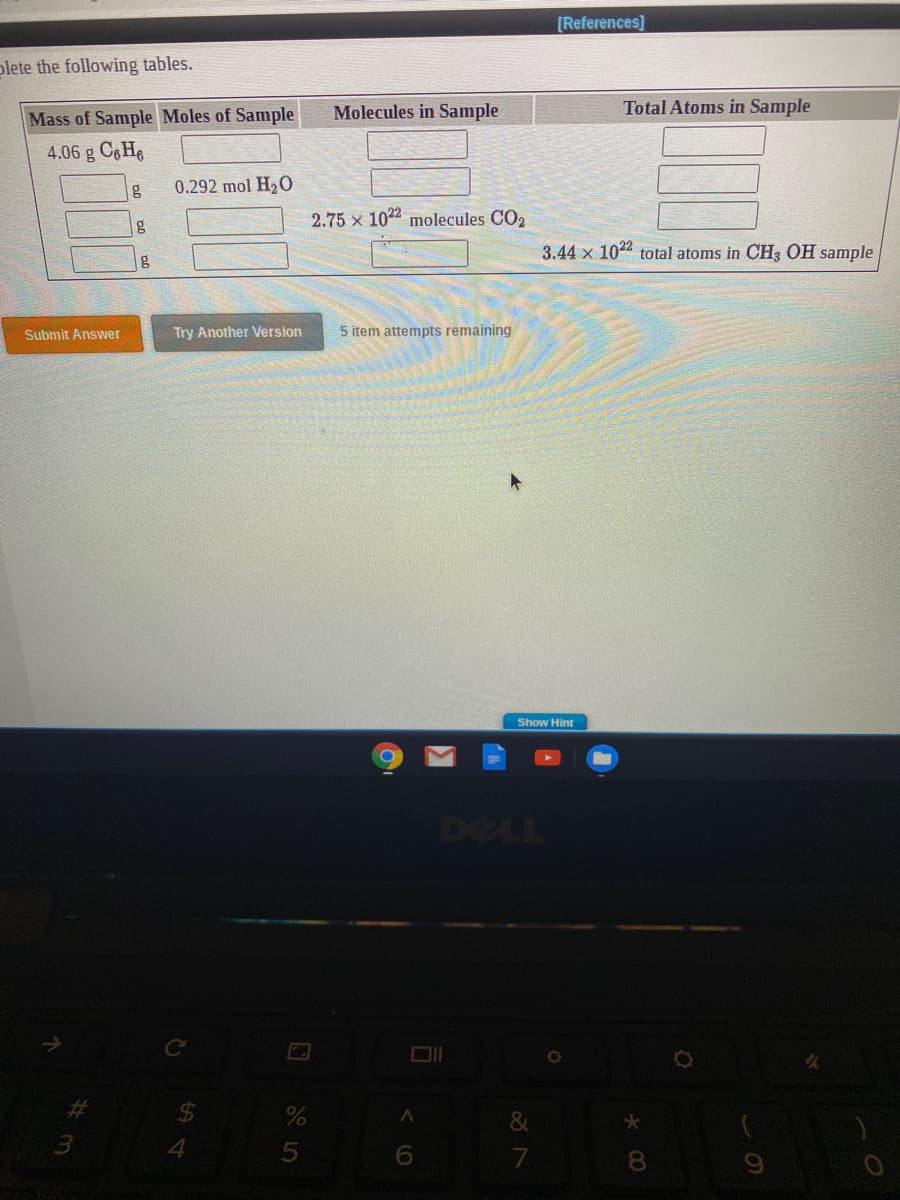
Transcribed Image Text:lete the following tables.
Molecules in Sample
Total Atoms in Sample
Mass of Sample Moles of Sample
Expert Solution
Step 1
Since you have posted a question with multiple sub-parts, we will solve first three subparts for you. To get the remaining sub-part solved please repost the complete question and mention the sub-parts to be solved.
Step 2
The following relations will help solve this problem.
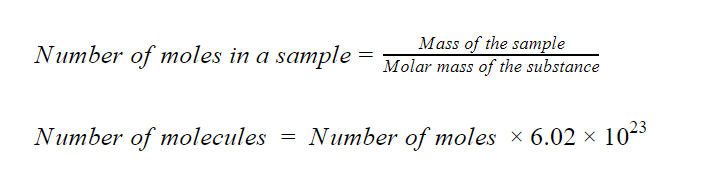
Step 3
1.
4.06 g C6H6
Molar mass of C6H6 = 6(Atomic mass of C) + 6(Atomic mass of H) = 6(12) + 6(1) g/mol = 78 g/mol
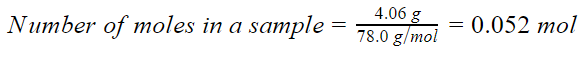
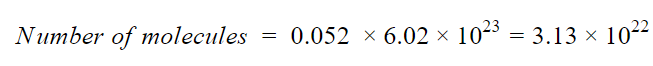
Each molecule of C6H6 contains 6 atoms of C and 6 atoms of H, thus there are total (6+6)= 12 atoms in one molecule of C6H6.
Thus,
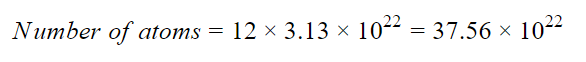
Step by step
Solved in 5 steps with 10 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax



Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER