Liquid methanol, CH;OH(1), is an important solvent in industry and can also be used as a fuel. Methanol may be synthesized in the gas state from carbon monoxide gas, CO(g), and hydrogen gas, H,(g), in a chemical reaction system that comes to equilibrium in a closed vessel at 225 °C. (a) Write the balanced chemical equation for the formation of methanol gas from carbon monoxide gas and hydrogen gas at 225 °C. (b) The equilibrium constant, K, for this reaction at 225 °C is 6.3 × 10¯³. Predict the relative concentration of the methanol gas when equilibrium is established. (c) Based on your answer for (b), predict whether this equilibrium system could generate large quantities of methanol. Explain your answer. (d) What would be the equilibrium constant for the reverse reaction?
Liquid methanol, CH;OH(1), is an important solvent in industry and can also be used as a fuel. Methanol may be synthesized in the gas state from carbon monoxide gas, CO(g), and hydrogen gas, H,(g), in a chemical reaction system that comes to equilibrium in a closed vessel at 225 °C. (a) Write the balanced chemical equation for the formation of methanol gas from carbon monoxide gas and hydrogen gas at 225 °C. (b) The equilibrium constant, K, for this reaction at 225 °C is 6.3 × 10¯³. Predict the relative concentration of the methanol gas when equilibrium is established. (c) Based on your answer for (b), predict whether this equilibrium system could generate large quantities of methanol. Explain your answer. (d) What would be the equilibrium constant for the reverse reaction?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 92QRT
Related questions
Question
Help me please
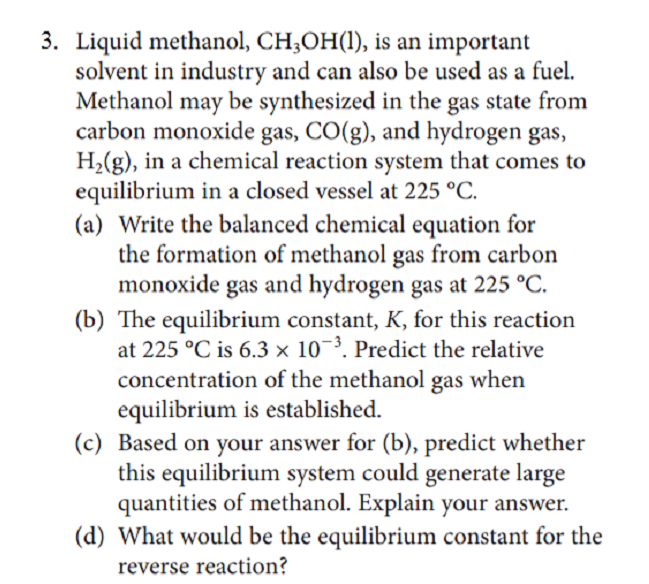
Transcribed Image Text:3. Liquid methanol, CH;OH(1), is an important
solvent in industry and can also be used as a fuel.
Methanol may be synthesized in the gas state from
carbon monoxide gas, CO(g), and hydrogen gas,
H2(g), in a chemical reaction system that comes to
equilibrium in a closed vessel at 225 °C.
(a) Write the balanced chemical equation for
the formation of methanol gas from carbon
monoxide gas and hydrogen gas at 225 °C.
(b) The equilibrium constant, K, for this reaction
at 225 °C is 6.3 × 10¬³. Predict the relative
concentration of the methanol gas when
equilibrium is established.
(c) Based on your answer for (b), predict whether
this equilibrium system could generate large
quantities of methanol. Explain your answer.
(d) What would be the equilibrium constant for the
reverse reaction?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
