*Xog pəpIAojd For reactions carried out under standard-state conditions, the equation AG=AH- TAS becomes AG" = H - TAS". Assuming AH" and AS are independent of temperature, one can derive the equation: K2 AH T2-T1. In %3D K1 where K1 and K2 are the equilibrium constants at T, and T2, respectively. Given that at 25.0°C, K̟ is 4.63 x 103 for the reaction N,O,(g) 5 2NO,(g) AH° =58.0 kJ/mol calculate the equilibrium constant at 40.0°C. K =
*Xog pəpIAojd For reactions carried out under standard-state conditions, the equation AG=AH- TAS becomes AG" = H - TAS". Assuming AH" and AS are independent of temperature, one can derive the equation: K2 AH T2-T1. In %3D K1 where K1 and K2 are the equilibrium constants at T, and T2, respectively. Given that at 25.0°C, K̟ is 4.63 x 103 for the reaction N,O,(g) 5 2NO,(g) AH° =58.0 kJ/mol calculate the equilibrium constant at 40.0°C. K =
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter5: Introduction To Chemical Equilibrium
Section: Chapter Questions
Problem 5.42E
Related questions
Question
100%
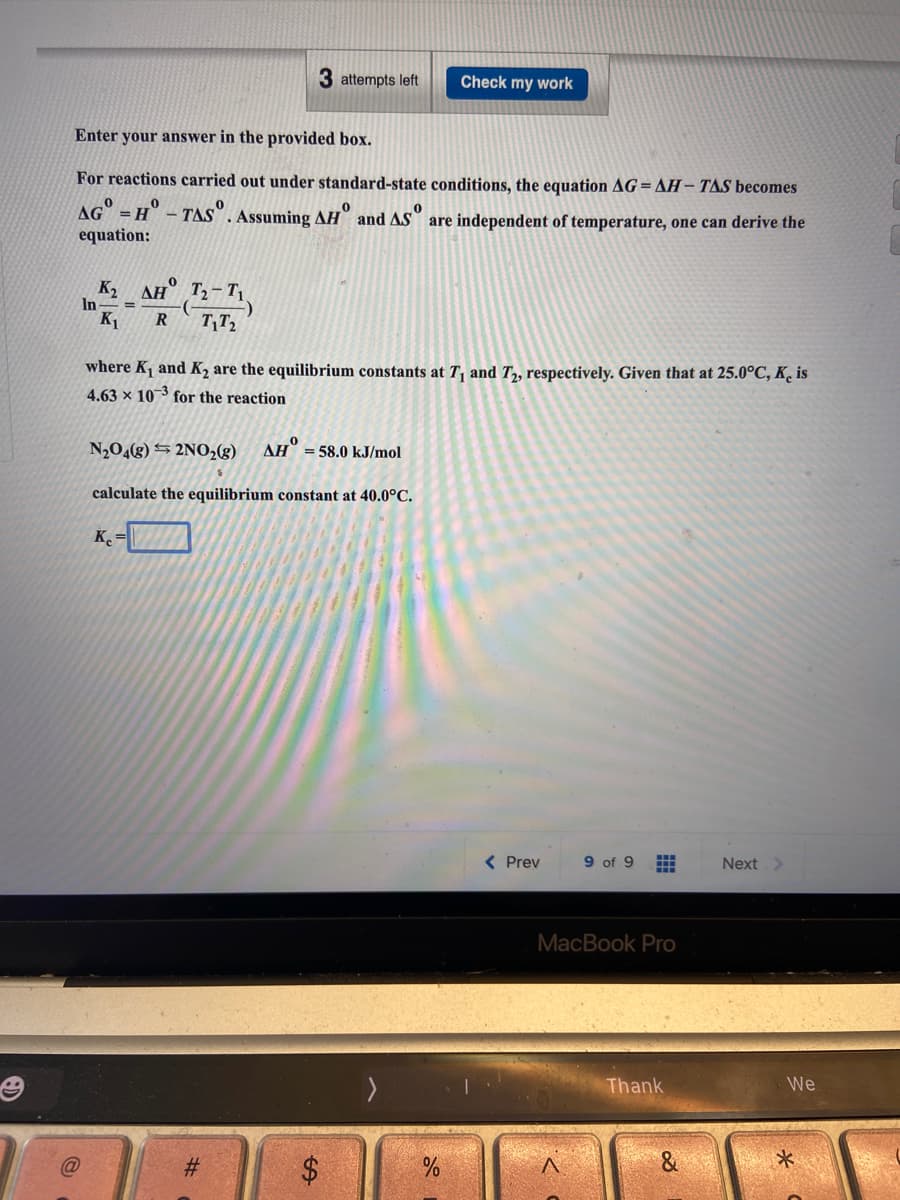
Transcribed Image Text:3 attempts left
Check my work
Enter your answer in the provided box.
For reactions carried out under standard-state conditions, the equation AG=AH- TAS becomes
AG = H° - TAS". Assuming AH" and AS" are independent of temperature, one can derive the
equation:
K2
AH T-T
In
K1
R
TT2
where K, and K, are the equilibrium constants at T, and T,, respectively. Given that at 25.0°C, K̟ is
4.63 × 10 3 for the reaction
N,O4(g) S 2NO2®)
AH
= 58.0 kJ/mol
calculate the equilibrium constant at 40.0°C.
K =
( Prev
9 of 9
Next >
MacBook Pro
Thank
We
%23
%24
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning