Liquid nitrogen trichloride is heated in a 1.75-L closed reaction vessel until it decomposes completely to gaseous elements. The resulting mixture exerts a pressure of 769. mmHg at 88.0 °C. What is the partial pressure of each gas in the container? (1 mmHg 1 torr.) Round each of your answers to 3 significant figures. = Part 1 of 2 Pressure of N2: Part 2 of 2 torr ☐ x10 ☑ Pressure of Cl₂: torr ☐ x10 ☑
Liquid nitrogen trichloride is heated in a 1.75-L closed reaction vessel until it decomposes completely to gaseous elements. The resulting mixture exerts a pressure of 769. mmHg at 88.0 °C. What is the partial pressure of each gas in the container? (1 mmHg 1 torr.) Round each of your answers to 3 significant figures. = Part 1 of 2 Pressure of N2: Part 2 of 2 torr ☐ x10 ☑ Pressure of Cl₂: torr ☐ x10 ☑
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.99PAE: 99 Pure gaseous nitrogen dioxide (NO2) cannot be obtained, because NO2dimerizes, or combines with...
Related questions
Question
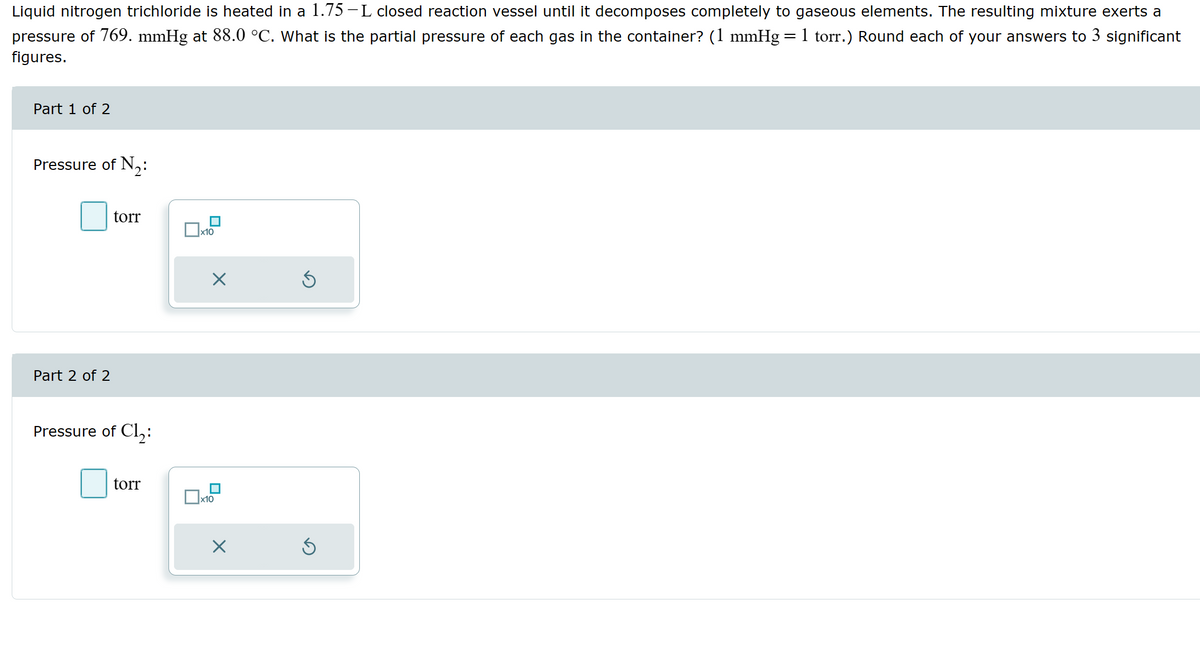
Transcribed Image Text:Liquid nitrogen trichloride is heated in a 1.75-L closed reaction vessel until it decomposes completely to gaseous elements. The resulting mixture exerts a
pressure of 769. mmHg at 88.0 °C. What is the partial pressure of each gas in the container? (1 mmHg 1 torr.) Round each of your answers to 3 significant
figures.
=
Part 1 of 2
Pressure of N2:
Part 2 of 2
torr
☐ x10
☑
Pressure of Cl₂:
torr
☐ x10
☑
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 1 steps with 1 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
