Listed below are the ionization energies for the first six ionizations of an imaginary element: 1st IE= 1,100 kJ/mol 2nd IE = 1,950 kJ/mol 3rd IE = 5,890 kJ/mol 4th IE = 6,740 kJ/mol 5th IE= 8,120 kJ/mol 6th IE= 9,050 kJ/mol Why is the 2nd ionization energy higher than the 1st ionization energy? Check the two that apply. The 2nd electron removed is a core electron and core electrons are closer to the nucleus and experience a higher effective nuclear charge. The attractive force between the 2nd electron and the nucleus is weaker and requires more energy to overcome than the attractive force between the 1st electron and the nucleus. The 2nd electron removed is a valence electron, but it is closer to the nucleus because the 1+ cation that it was removed from is smaller than the neutral atom. The attractive force between the 2nd electron and the nucleus is stronger and requires more energy to overcome than the attractive force between the 1st electron and the nucleus.
Listed below are the ionization energies for the first six ionizations of an imaginary element: 1st IE= 1,100 kJ/mol 2nd IE = 1,950 kJ/mol 3rd IE = 5,890 kJ/mol 4th IE = 6,740 kJ/mol 5th IE= 8,120 kJ/mol 6th IE= 9,050 kJ/mol Why is the 2nd ionization energy higher than the 1st ionization energy? Check the two that apply. The 2nd electron removed is a core electron and core electrons are closer to the nucleus and experience a higher effective nuclear charge. The attractive force between the 2nd electron and the nucleus is weaker and requires more energy to overcome than the attractive force between the 1st electron and the nucleus. The 2nd electron removed is a valence electron, but it is closer to the nucleus because the 1+ cation that it was removed from is smaller than the neutral atom. The attractive force between the 2nd electron and the nucleus is stronger and requires more energy to overcome than the attractive force between the 1st electron and the nucleus.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter7: The Structure Of Atoms And Periodic Trends
Section: Chapter Questions
Problem 64GQ: Answer the questions below concerning ground state electron configurations. (a) What element has the...
Related questions
Question

Transcribed Image Text:Listed below are the ionization energies for the first six ionizations of an imaginary element:
1st IE= 1,100 kJ/mol
2nd IE = 1,950 kJ/mol
3rd IE = 5,890 kJ/mol
4th IE= 6,740 kJ/mol
5th IE= 8,120 kJ/mol
6th IE= 9,050 kJ/mol
Why is the 2nd ionization energy higher than the 1st ionization energy? Check the two that apply.
The 2nd electron removed is a core electron and core electrons are closer to the nucleus and experience a
higher effective nuclear charge.
The attractive force between the 2nd electron and the nucleus is weaker and requires more energy to
overcome than the attractive force between the 1st electron and the nucleus.
The 2nd electron removed is a valence electron, but it is closer to the nucleus because the 1+ cation that it
was removed from is smaller than the neutral atom.
The attractive force between the 2nd electron and the nucleus is stronger and requires more energy to
overcome than the attractive force between the 1st electron and the nucleus.
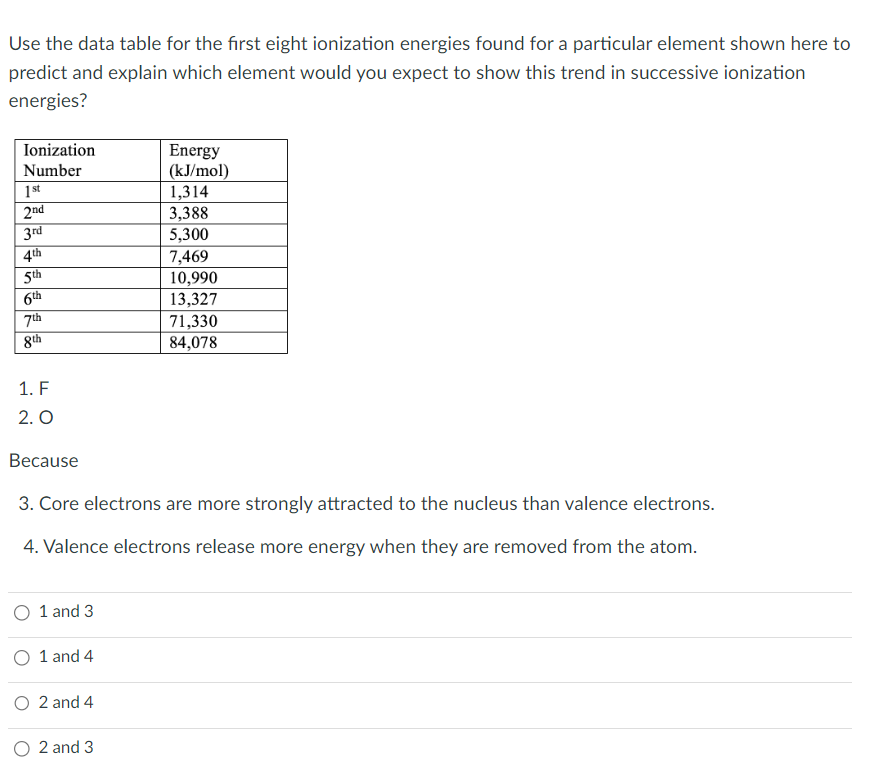
Transcribed Image Text:Use the data table for the first eight ionization energies found for a particular element shown here to
predict and explain which element would you expect to show this trend in successive ionization
energies?
Ionization
Number
1st
2nd
3rd
4th
5th
6th
7th
8th
1. F
2. O
Because
1 and 3
1 and 4
3. Core electrons are more strongly attracted to the nucleus than valence electrons.
4. Valence electrons release more energy when they are removed from the atom.
O2 and 4
Energy
(kJ/mol)
O 2 and 3
1,314
3,388
5,300
7,469
10,990
13,327
71,330
84,078
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning