مع مسن NO₂ NaCN CN NO₂ Although aromatic substitution reactions usually occur by an electrophilic mechanism, aryl halides that have electron-withdrawing substituents can also undergo a nucleophilic substitution reaction, termed SAr. The electron-withdrawing substituent(s) must be oriented ortho and/or para to the halogen. The reaction proceeds by nucleophilic attack of the nucleophile on the ring carbon bound to the halogen leaving group, an intermediate carbanion is formed which is termed a Meisenheimer complex. The Meisenheimer complex is stabilized via resonance by the electron-withdrawing groups. Expulsion of the halogen restores aromaticity to the ring and completes the reaction. Draw curved arrows to show the movement of electrons this step of the mechanism. Arrow-pushing Instructions 20 →XT NC CI NC gr - gr
مع مسن NO₂ NaCN CN NO₂ Although aromatic substitution reactions usually occur by an electrophilic mechanism, aryl halides that have electron-withdrawing substituents can also undergo a nucleophilic substitution reaction, termed SAr. The electron-withdrawing substituent(s) must be oriented ortho and/or para to the halogen. The reaction proceeds by nucleophilic attack of the nucleophile on the ring carbon bound to the halogen leaving group, an intermediate carbanion is formed which is termed a Meisenheimer complex. The Meisenheimer complex is stabilized via resonance by the electron-withdrawing groups. Expulsion of the halogen restores aromaticity to the ring and completes the reaction. Draw curved arrows to show the movement of electrons this step of the mechanism. Arrow-pushing Instructions 20 →XT NC CI NC gr - gr
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter14: Rotational And Vibrational Spectroscopy
Section: Chapter Questions
Problem 14.5E: What is the energy of light having each characteristic? a v=6.031014s1 b =9.27nm c v=4320cm1 d =5.69
Related questions
Question
100%
One picture is the instructions and the other is the one that needs to be solved.
(Draw curved arrows to show the movement of electrons in this step of the mechanism.)

Transcribed Image Text:من مع
NO₂
NA
NaCN
Although aromatic substitution reactions usually occur by an electrophilic mechanism, aryl halides that have electron-withdrawing substituents can also undergo a nucleophilic substitution reaction, termed SNAr. The electron-withdrawing
substituent(s) must be oriented ortho and/or para to the halogen. The reaction proceeds by nucleophilic attack of the nucleophile on the ring carbon bound to the halogen leaving group, an intermediate carbanion is formed which is termed
a Meisenheimer complex. The Meisenheimer complex is stabilized via resonance by the electron-withdrawing groups. Expulsion of the halogen restores aromaticity to the ring and completes the reaction.
Draw curved arrows to show the movement of electrons in this step of the mechanism.
Arrow-pushing Instructions
х ш
CN
NC
CI
NC
CI
gr - gr
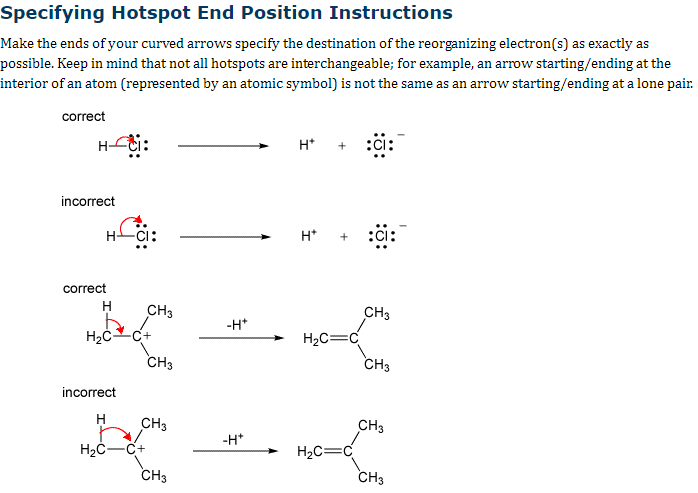
Transcribed Image Text:Specifying Hotspot End Position Instructions
Make the ends of your curved arrows specify the destination of the reorganizing electron(s) as exactly as
possible. Keep in mind that not all hotspots are interchangeable; for example, an arrow starting/ending at the
interior of an atom (represented by an atomic symbol) is not the same as an arrow starting/ending at a lone pair.
correct
Hoti:
incorrect
correct
HCI:
H
H₂C C+
incorrect
H
CH3
H₂C-C+
CH3
CH3
CH3
-H*
-H*
H+
H*
H₂C=C
H₂C=
:CI:
CH3
CH3
CH3
CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,