Macmillan Learning You decide it is time to clean your pool since summer is quickly approaching. Your pool maintenance guide specifies that the chlorine, Cl₂, concentration of the pool should be between 1 and 3 ppm. In order to determine if your pool is safe to swim in, you send a sample of pool water to a chemist for analysis of the Cl₂ content. The chemist reports a chlorine concentration of 1.86 x 10-5 M. Convert the concentration of Cl₂ to parts per million (ppm). concentration: ppm
Macmillan Learning You decide it is time to clean your pool since summer is quickly approaching. Your pool maintenance guide specifies that the chlorine, Cl₂, concentration of the pool should be between 1 and 3 ppm. In order to determine if your pool is safe to swim in, you send a sample of pool water to a chemist for analysis of the Cl₂ content. The chemist reports a chlorine concentration of 1.86 x 10-5 M. Convert the concentration of Cl₂ to parts per million (ppm). concentration: ppm
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter1: Chemistry And Measurement
Section: Chapter Questions
Problem 1.37QP: A 15.5 g sample of sodium carbonate is added to a solution of acetic acid weighing 19.7 g. The two...
Related questions
Question
100%
I have submitted this question before however the answer given to me is incorrect. Please

Transcribed Image Text:Macmillan Learning
You decide it is time to clean your pool since summer is quickly approaching. Your pool maintenance guide specifies that the
chlorine, Cl₂, concentration of the pool should be between 1 and 3 ppm. In order to determine if your pool is safe to swim in,
you send a sample of pool water to a chemist for analysis of the Cl, content. The chemist reports a chlorine concentration of
1.86 x 10-5 M.
Convert the concentration of Cl₂ to parts per million (ppm).
concentration:
ppm
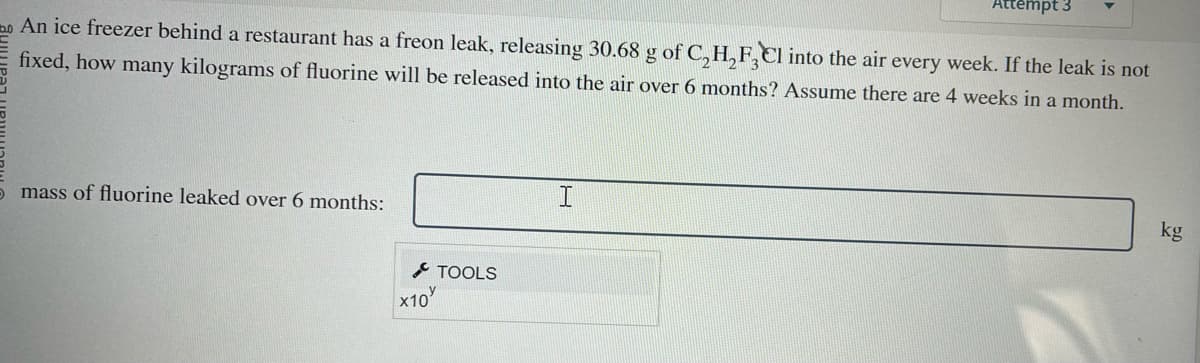
Transcribed Image Text:An ice freezer behind a restaurant has a freon leak, releasing 30.68 g of C₂H₂F3Cl into the air every week. If the leak is not
fixed, how many kilograms of fluorine will be released into the air over 6 months? Assume there are 4 weeks in a month.
5 mass of fluorine leaked over 6 months:
x10
TOOLS
Attempt 3
I
kg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning