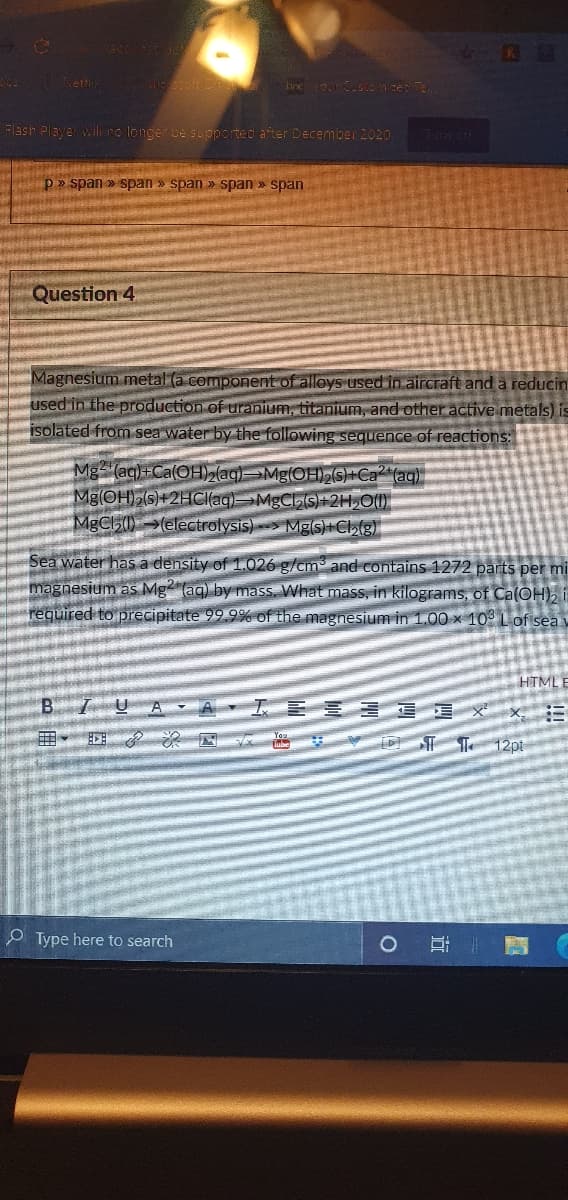
Magnesium metal (a component of alloys used in aircraft and a reducir used in the production of uranium, titanium, and other active metals) i isolated from sea water by the following sequence of reactions: Mg (aq)+Ca(OH)2(aq)=¬Mg(OH)S)+Ca² (aq) Mg(OH) (s)+2HCI(ag)MgCs)+2H,O() MgCh)(electrolysis)> Mg(s)+Ch(g) Sea water has a density of 1.026 g/cm and contains 1272 parts per m magnesium as Mg (ag) by mass, What mass, in kilograms, of Ca(OH), required to precipitate 99.9% of the magnesium in 1.00 x 10 Lof sea
Magnesium metal (a component of alloys used in aircraft and a reducir used in the production of uranium, titanium, and other active metals) i isolated from sea water by the following sequence of reactions: Mg (aq)+Ca(OH)2(aq)=¬Mg(OH)S)+Ca² (aq) Mg(OH) (s)+2HCI(ag)MgCs)+2H,O() MgCh)(electrolysis)> Mg(s)+Ch(g) Sea water has a density of 1.026 g/cm and contains 1272 parts per m magnesium as Mg (ag) by mass, What mass, in kilograms, of Ca(OH), required to precipitate 99.9% of the magnesium in 1.00 x 10 Lof sea
Chapter5: Gases
Section: Chapter Questions
Problem 161IP: In the presence of nitric acid, UO2+ undergoes a redox process. It is converted to UO22+ and nitric...
Related questions
Question
Transcribed Image Text:eti
Flash Player wilno longer be supported after December 2020
Tum off
p » span » Span » span » span » span
Question 4
Magnesium metal (a component of alloys used in aircraft and a reducin
used in the production of uranium, titanium, and other active metals) is
isolated from sea water by the following sequence of reactions:
Mg (ag)+Ca(OH, (ag)Mg(OHS)+Ca (ag)
Mg(OH)2(s)+2HC(ag)MgCS)+2H,O(1)
MgCl5()→(electrolysis)> Mgs)+Cl,(g)
Sea water has a density of 1.026 g/cm and contains 1272 parts per mi
magnesium as Mg (aq) by mass, What mass, in kilograms, of Ca(OH), i
required to precipitate 99.9% of the magnesium in 1.00 × 10° L of sea v
HTML E
工
12pt
O Type here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning