Malonic acid (Ka1 = 1.42 x 10–3, Ka2 = 2.01 x 10–6) can exist as one of the 3 forms shown below. What is the pH of a 0.1000 M solution of malonate
Malonic acid (Ka1 = 1.42 x 10–3, Ka2 = 2.01 x 10–6) can exist as one of the 3 forms shown below. What is the pH of a 0.1000 M solution of malonate
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
ChapterNW4: Nomenclature Worksheet 4: Carbonyl Compounds
Section: Chapter Questions
Problem 8CTQ
Related questions
Question
Malonic acid (Ka1 = 1.42 x 10–3, Ka2 = 2.01 x 10–6) can exist as one of the 3 forms shown below.
What is the pH of a 0.1000 M solution of malonate?
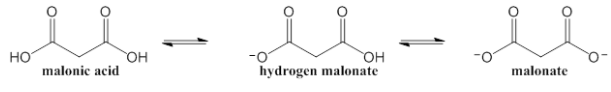
Transcribed Image Text:hydregen malonate
HO
он
hydrogen malonate
malonic acid
HO
malonate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning