Mass of a whole tablet: 3.2338g Mass of portion of tablet used Volume of flask Initial Pressure Maximum Pressure ΔΡ Trial 1 2.083g 138.5 mL 105.89 KPa 126.92 K Pa 21.03 Trial 2 2.054g 138.5 mL 104.81 KPa 125.27 KPq 20.46 Trial 3 2.061g 138.5 mL 105.23 кра 125.54 k Pa 20.31
Mass of a whole tablet: 3.2338g Mass of portion of tablet used Volume of flask Initial Pressure Maximum Pressure ΔΡ Trial 1 2.083g 138.5 mL 105.89 KPa 126.92 K Pa 21.03 Trial 2 2.054g 138.5 mL 104.81 KPa 125.27 KPq 20.46 Trial 3 2.061g 138.5 mL 105.23 кра 125.54 k Pa 20.31
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 6QAP: A tank is filled with gas to a pressure of 875 mm Hg at 25C. The gas is transferred without loss to...
Related questions
Question
Please calculate problems 2, 3, and 4 using the data table provided if needed. For problem 2 the moles of CO2 is 1.20 x 10^-3 mol
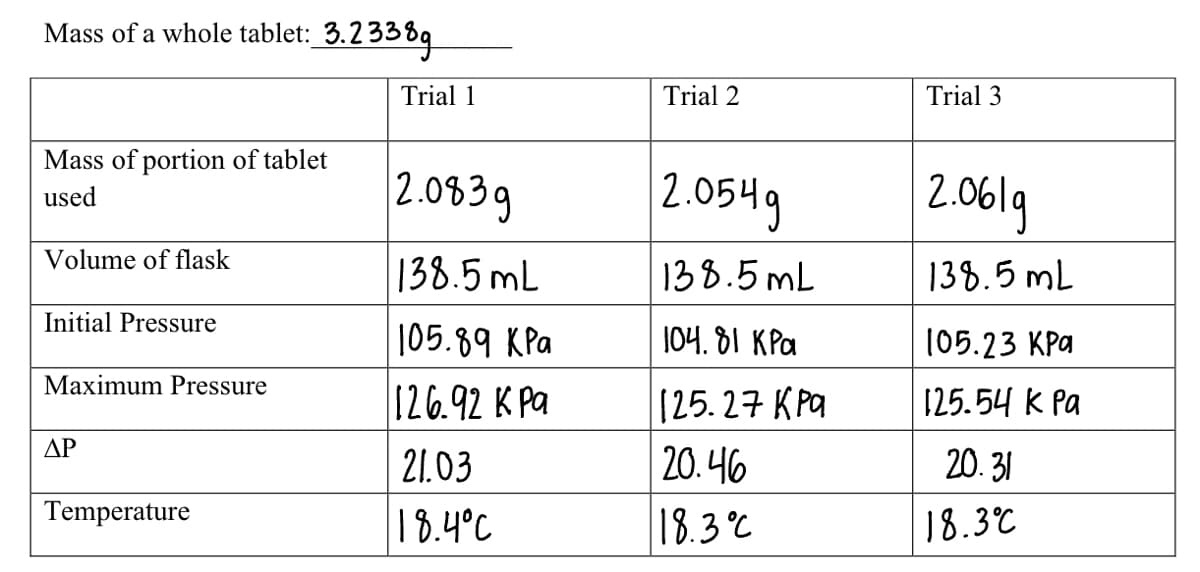
Transcribed Image Text:Mass of a whole tablet: 3.2338g
Mass of portion of tablet
used
Volume of flask
Initial Pressure
Maximum Pressure
ΔΡ
Temperature
Trial 1
2.0839
138.5 mL
105.89 KPa
126.92 K Pa
21.03
18.4°C
Trial 2
2.0549
138.5mL
104.81 KPa
[25.27 кра
20.46
18.3°C
Trial 3
2.061g
138.5 mL
105.23 KPa
125.54 k Pa
20.31
18.3°C
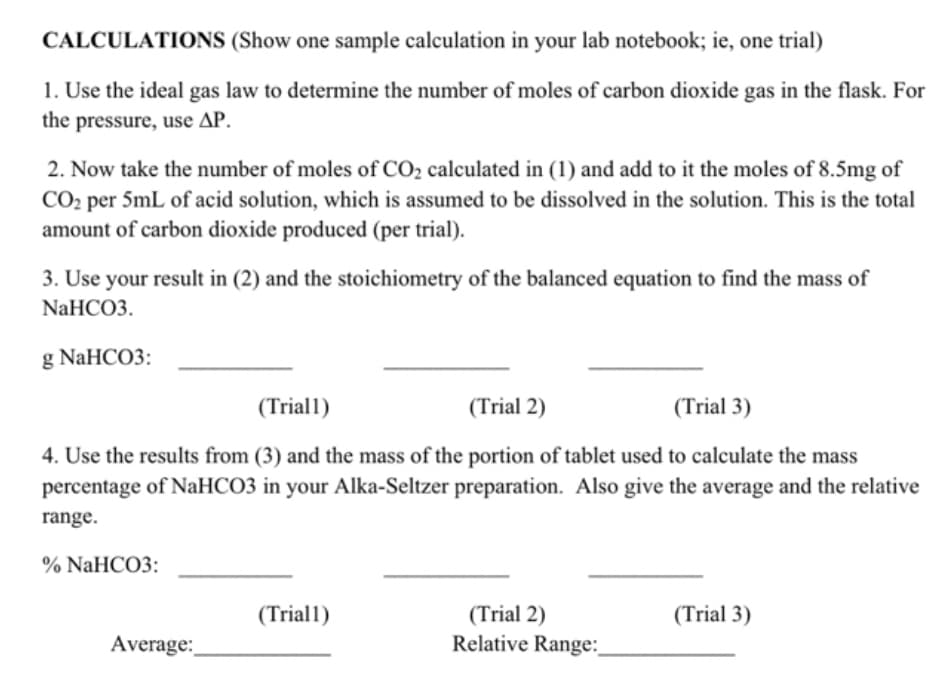
Transcribed Image Text:CALCULATIONS (Show one sample calculation in your lab notebook; ie, one trial)
1. Use the ideal gas law to determine the number of moles of carbon dioxide gas in the flask. For
the pressure, use AP.
2. Now take the number of moles of CO₂ calculated in (1) and add to it the moles of 8.5mg of
CO₂ per 5mL of acid solution, which is assumed to be dissolved in the solution. This is the total
amount of carbon dioxide produced (per trial).
3. Use your result in (2) and the stoichiometry of the balanced equation to find the mass of
NaHCO3.
g NaHCO3:
(Triall)
(Trial 2)
(Trial 3)
4. Use the results from (3) and the mass of the portion of tablet used to calculate the mass
percentage of NaHCO3 in your Alka-Seltzer preparation. Also give the average and the relative
range.
% NaHCO3:
Average:
(Triall)
(Trial 2)
Relative Range:
(Trial 3)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER