Match each of the following transition metals to the type of method used to isolate it from its ore. Co Mn Clear All Reduction of oxide Sulfide roasted to oxide and then reduced
Match each of the following transition metals to the type of method used to isolate it from its ore. Co Mn Clear All Reduction of oxide Sulfide roasted to oxide and then reduced
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter8: Bonding In Transition Metal Compounds And Coordination Complexes
Section: Chapter Questions
Problem 34P
Related questions
Question
plz with detail explanation do both or else skip
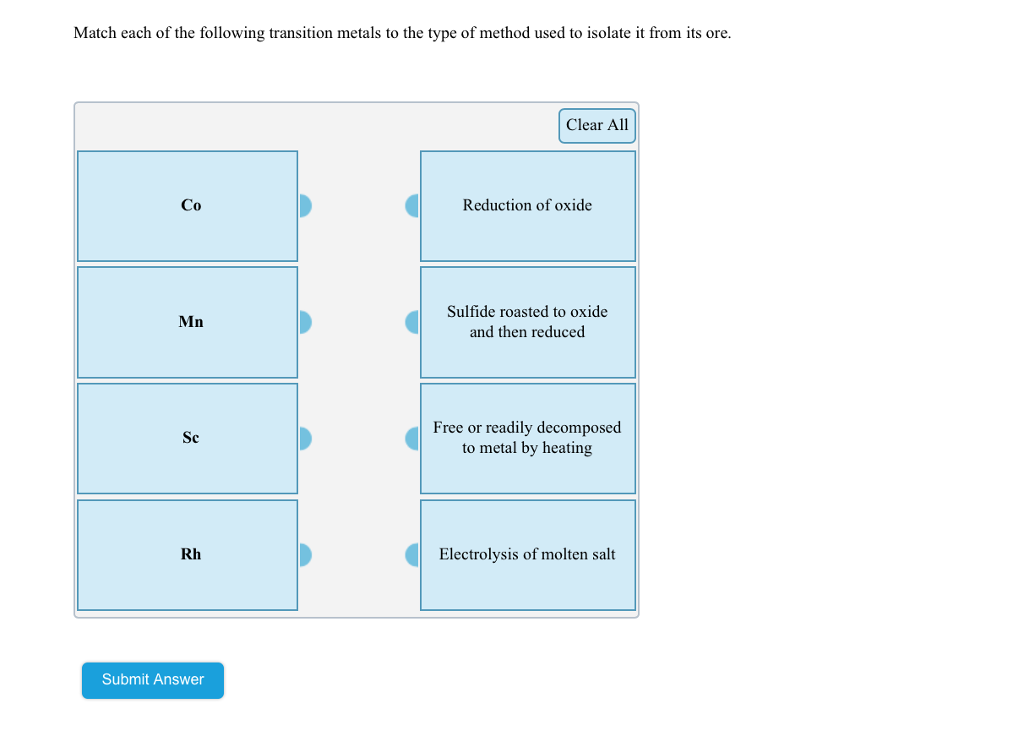
Transcribed Image Text:Match each of the following transition metals to the type of method used to isolate it from its ore.
Co
Mn
Sc
Rh
Submit Answer
Clear All
Reduction of oxide
Sulfide roasted to oxide
and then reduced
Free or readily decomposed
to metal by heating
Electrolysis of molten salt
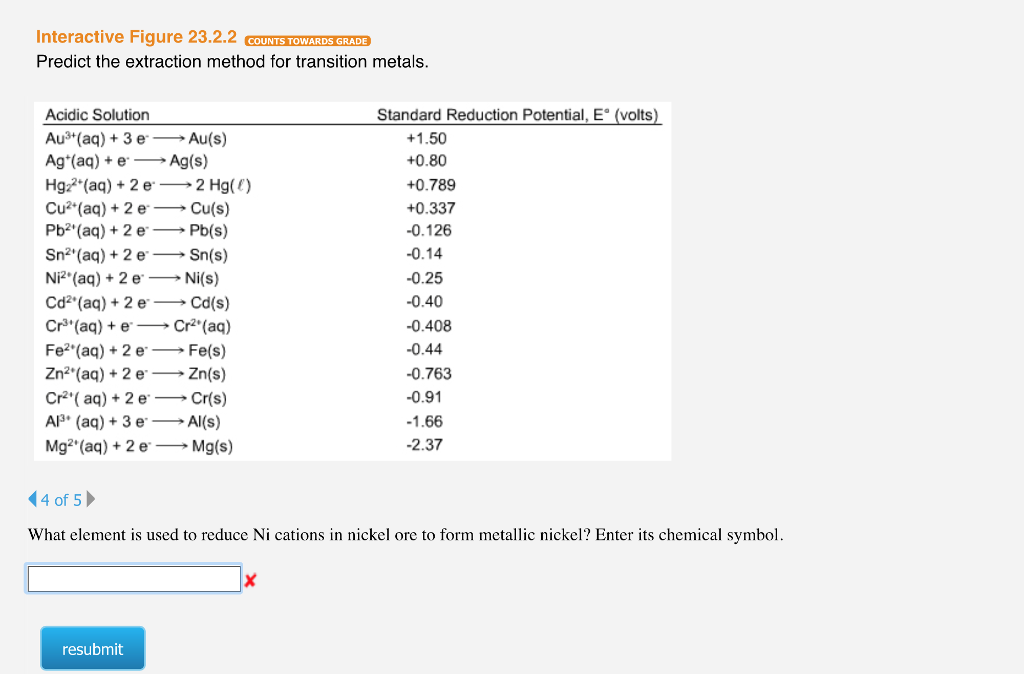
Transcribed Image Text:Interactive Figure 23.2.2 COUNTS TOWARDS GRADE
Predict the extraction method for transition metals.
Acidic Solution
Au³+ (aq) + 3 e→→→→ Au(s)
Ag+ (aq) + e
→ Ag(s)
Hg₂+ (aq) + 2 e →→→ 2 Hg()
Cu²+ (aq) + 2 e
· Cu(s)
Pb²¹(aq) + 2 e
Pb(s)
Sn²¹ (aq) + 2 e
Sn(s)
Ni² (aq) + 2 e
Cd²(aq) + 2 e
Cr³(aq) + e
Fe²(aq) + 2 e
Zn² (aq) + 2 e
Ni(s)
Cd(s)
Cr²(aq)
resubmit
Fe(s)
Zn(s)
Cr²¹(aq) + 2 e →→→ Cr(s)
Al³+ (aq) + 3 e-Al(s)
Mg2+ (aq) + 2 e →→→ Mg(s)
Standard Reduction Potential, E° (volts)
+1.50
+0.80
+0.789
+0.337
-0.126
-0.14
-0.25
-0.40
-0.408
-0.44
-0.763
-0.91
-1.66
-2.37
4 of 5
What element is used to reduce Ni cations in nickel ore to form metallic nickel? Enter its chemical symbol.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning