Materials at 20°C for light with a vacuum wavelength of 589 nm. Material n Material n Material benzene 1.501 diamond plexiglas carbon disulfide 1.628 fluorite quartz (crystalline) carbon tetrachloride 1.461 glass (crown) quartz (fused) ethanol 1.361 glass (flint) sodium chloride glycerine 1.473 ice (0°C) zircon water (fresh) 1.333 polystyrene air 2.419 1.434 1.52 1.66 1.309 1.49 n 1.51 1.544 1.458 1.544 1.923 1.00029
Materials at 20°C for light with a vacuum wavelength of 589 nm. Material n Material n Material benzene 1.501 diamond plexiglas carbon disulfide 1.628 fluorite quartz (crystalline) carbon tetrachloride 1.461 glass (crown) quartz (fused) ethanol 1.361 glass (flint) sodium chloride glycerine 1.473 ice (0°C) zircon water (fresh) 1.333 polystyrene air 2.419 1.434 1.52 1.66 1.309 1.49 n 1.51 1.544 1.458 1.544 1.923 1.00029
Physics for Scientists and Engineers
10th Edition
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter37: Diffraction Patterns And Polarization
Section: Chapter Questions
Problem 12P: A heliumneon laser emits light that has a wavelength of 632.8 nm. The circular aperture through...
Related questions
Question
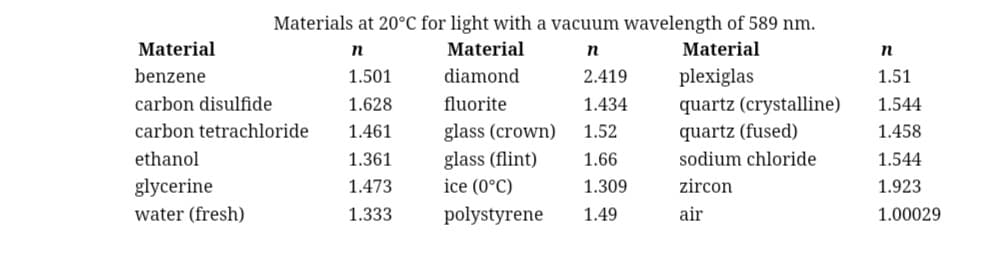
Chapter 25: Problem 11: Consider the internal reflection of light at the interface between water and ice. For convenience, the index of refraction for a variety of materials is provided below.
a) What is the minimum angle, in degrees, at which you will get total internal reflection at this interface?
Transcribed Image Text:Materials at 20°C for light with a vacuum wavelength of 589 nm.
Material
n
Material
n
Material
benzene
1.501
diamond
plexiglas
carbon disulfide
1.628
fluorite
quartz (crystalline)
carbon tetrachloride 1.461
glass (crown)
quartz (fused)
ethanol
1.361
glass (flint)
sodium chloride
glycerine
1.473
ice (0°C)
zircon
water (fresh)
1.333
polystyrene
air
2.419
1.434
1.52
1.66
1.309
1.49
n
1.51
1.544
1.458
1.544
1.923
1.00029
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 1 steps with 1 images

Recommended textbooks for you

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning