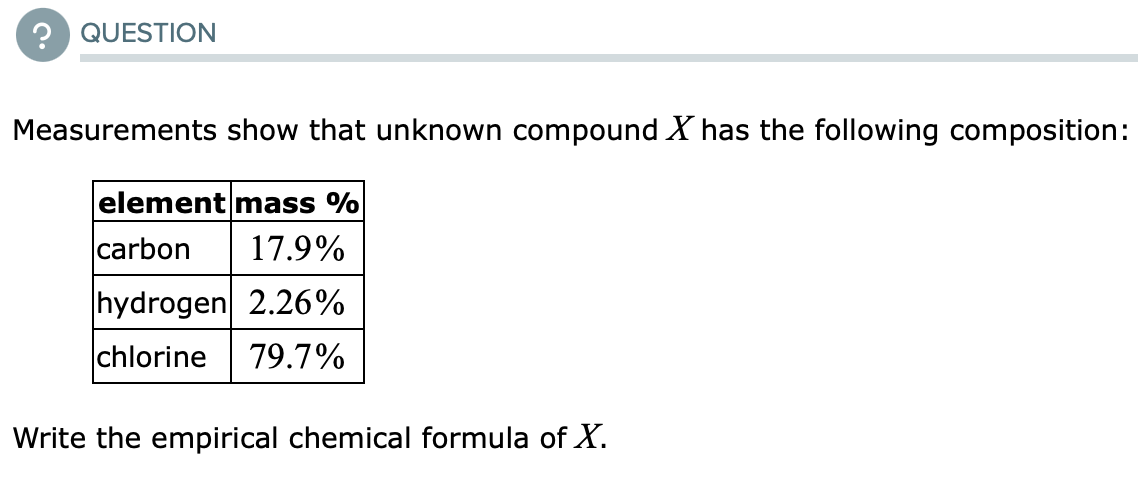
Measurements show that unknown compound X has the following composition: element mass % carbon 17.9% hydrogen 2.26% chlorine 79.7% Write the empirical chemical formula of X.
Measurements show that unknown compound X has the following composition: element mass % carbon 17.9% hydrogen 2.26% chlorine 79.7% Write the empirical chemical formula of X.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter3: Composition Of Substances And Solutions
Section: Chapter Questions
Problem 37E: Determine the empirical formulas for compounds with the following percent compositions: 15.8% carbon...
Related questions
Question
I am somewhat lost on step four.
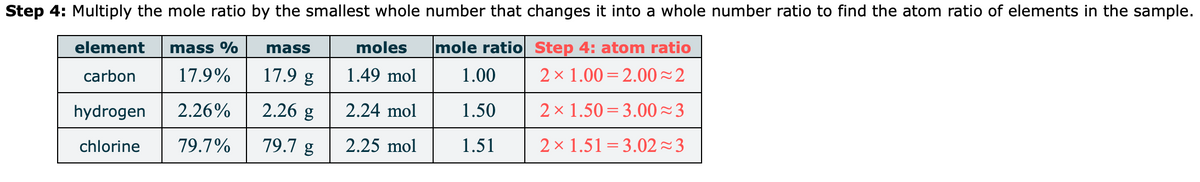
Transcribed Image Text:Step 4: Multiply the mole ratio by the smallest whole number that changes it into a whole number ratio to find the atom ratio of elements in the sample.
element
mass %
mass
moles
mole ratio Step 4: atom ratio
carbon
17.9%
17.9 g
1.49 mol
1.00
2 x 1.00 = 2.00~2
hydrogen
2.26%
2.26 g
2.24 mol
1.50
2 x 1.50 = 3.00~3
chlorine
79.7%
79.7 g
2.25 mol
1.51
2 x 1.51=3.02 23
Transcribed Image Text:? QUESTION
Measurements show that unknown compound X has the following composition:
element mass %
carbon
17.9%
hydrogen 2.26%
chlorine
79.7%
Write the empirical chemical formula of X.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co