Melting point is Pressure dependent. Choose... TWo different pure compounds can have the same melting point. Choose... Melting point is a physical property. Choose.. Tou need to use acetanilide in a reaction which requires a pure sample. You have two containers; container A has acetanilide with a melting point of 108-113°C and container B has acetanilide with a melting point of 112-114 °C. Which container should you use for the reaction? Explain. Normal BIIIU X2| X² | → EEE fx| IDI e E lli Ix
Melting point is Pressure dependent. Choose... TWo different pure compounds can have the same melting point. Choose... Melting point is a physical property. Choose.. Tou need to use acetanilide in a reaction which requires a pure sample. You have two containers; container A has acetanilide with a melting point of 108-113°C and container B has acetanilide with a melting point of 112-114 °C. Which container should you use for the reaction? Explain. Normal BIIIU X2| X² | → EEE fx| IDI e E lli Ix
Chapter82: Physical Constants Of Liquids: The Boiling Point And Density
Section: Chapter Questions
Problem 5P
Related questions
Question
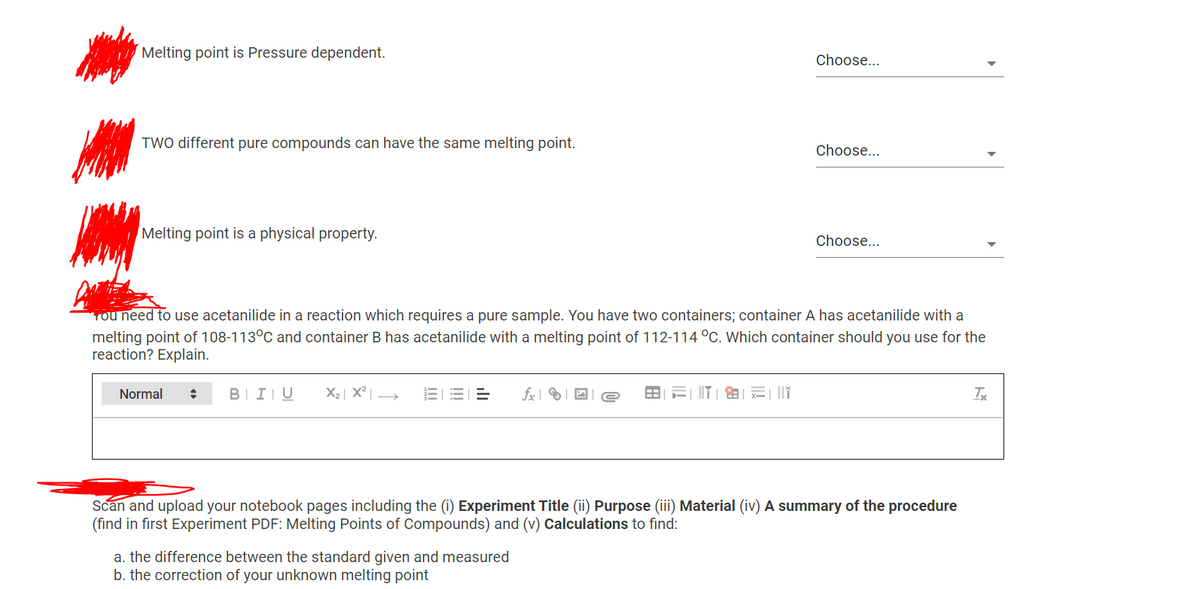
Transcribed Image Text:Melting point is Pressure dependent.
Choose...
TWO different pure compounds can have the same melting point.
Choose...
Melting point is a physical property.
Choose...
Tou need to use acetanilide in a reaction which requires a pure sample. You have two containers; container A has acetanilide with a
melting point of 108-113°C and container B has acetanilide with a melting point of 112-114 °C. Which container should you use for the
reaction? Explain.
Normal
BIIIU
X2 | X² |
fx
Tx
Scan and upload your notebook pages including the (i) Experiment Title (ii) Purpose (iii) Material (iv) A summary of the procedure
(find in first Experiment PDF: Melting Points of Compounds) and (v) Calculations to find:
a. the difference between the standard given and measured
b. the correction of your unknown melting point
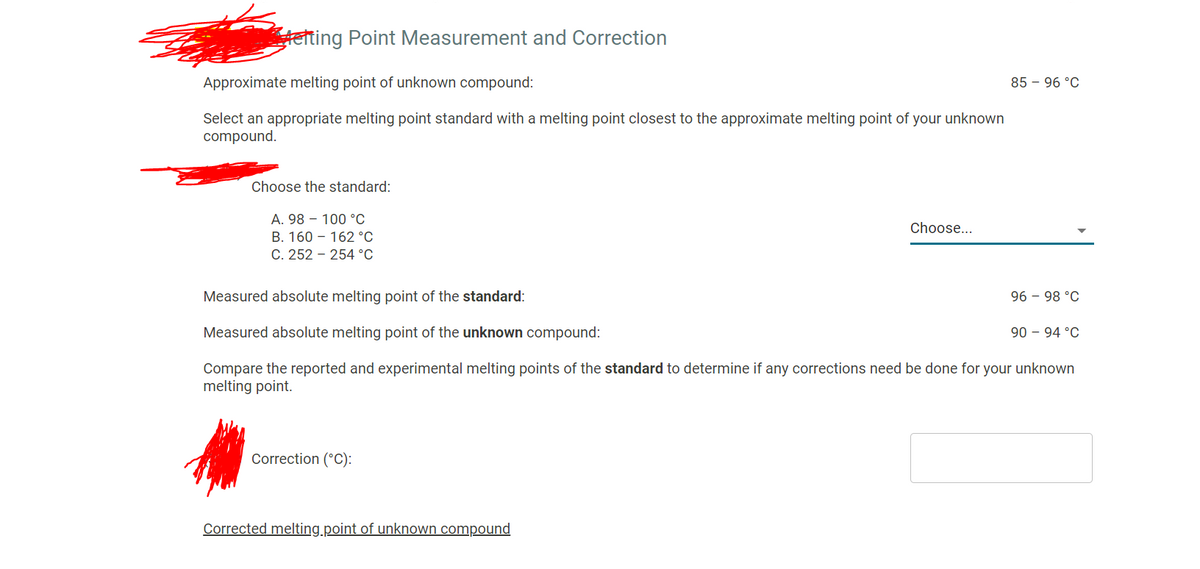
Transcribed Image Text:teting Point Measurement and Correction
Approximate melting point of unknown compound:
85 – 96 °C
Select an appropriate melting point standard with a melting point closest to the approximate melting point of your unknown
compound.
Choose the standard:
A. 98 – 100 °c
Choose...
B. 160 – 162 °C
C. 252 - 254 °C
Measured absolute melting point of the standard:
96 - 98 °C
Measured absolute melting point of the unknown compound:
90 – 94 °C
Compare the reported and experimental melting points of the standard to determine if any corrections need be done for your unknown
melting point.
Correction (°C):
Corrected melting.point of unknown compound
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

