Q: Increasing reagent concentration makes the change in pAg larger.
A: we know, pAg = -log[Ag+]. so with increasing the concentration of reagents,pAg decreases. Option C…
Q: In thin layer chromatography the initial spot is placed: A. on a pencil line B. four centimeters…
A: Thin layer chromatography is a type of chromatography.
Q: How do I find the standard deviation of the molaR CONCENTRATION and the relative standard deviation?…
A: A numerical problem based on standard deviation, which is to be accomplished.
Q: The same chloride analysis but using a new method for which the standard deviation was not known,…
A:
Q: I'm not sure how to approach this problem because the ruler on the chromatogram starts at 2.
A: Rf value in chromatography, is the ratio of distance travelled by solute to distance travelled by…
Q: True or false. A primary standard solid must be weighed accurately and diluted to an accurately…
A: Analytical chemistry.
Q: Which statement is valid when the multiple extraction and single extraction is compared.
A:
Q: In the vapor deposition method, solids are usually converted into solutions using their solvents. *…
A: A question based on general chemistry that is to be accomplished.
Q: A supporting electrolyte is usually added to the analyte solution to minimize solution…
A: 1. SOLUTION - The correct option is (a) minimize solution resistance According to the question -…
Q: Nitrogen Content Assay Method I is also known as? Distillation Micrometric Method Macromethod…
A: Nitrogen Content Assay Method I is also known as?
Q: Calculate the mean and standard deviation for the following set of analytical results: 34.65,…
A: Please find your solution below : Mean is calculated by taking the sum of all the values of data and…
Q: Give the standard deviation of these three molaritys. 0.0366 0.0373 0.0370
A: Standard deviation: Standard deviation tell about the variability(error) in data set. If standard…
Q: What type of components /mixtures can be separated by chromatography
A: Chromatography is a method which usually separate components of a homogeneous mixture based on the…
Q: he most convenient way of expressing the random error, regardless of the concentration or weight of…
A: The most convenient way of expressing the random error, regardless of the concentration or weight of…
Q: A compound X is to be determined by UV/Visible spectrophotometry. A calibration curve is constructed…
A: The light absorbed by the solution is known as absorbance.
Q: (a) (Apply fractions as (b) (Use the lowest pos (c) (Apply fractions as r
A: According to the question, we need to write the balanced formation equation at standard conditions…
Q: In a gravimetric chloride analysis, it was found that 0.82g AgCl and 0.80g AgCl was obtained from an…
A: Moles = mass / molecular mass molecular mass of AgCl = 108 + 35.5 = 143.5 => moles of AgCl in…
Q: II. lodometry II. Permanganate IV. Dichromate
A: Indicators can be defined as the substances that change colour when they are added to basic or…
Q: A titration was carried out to determine the volume of base needed to neutralize an acid. Five runs…
A: The neutralization reaction or the acid-base reaction is often employed as the basis for determining…
Q: How do you find the standard deviation with these calculations?
A: If n is the number of observations, m is the mean value of all the observation and the set of the…
Q: Calculate the following. i) The slope (m) and the standard deviation of the slope. ii) The…
A: The amount of a substance residing in a solution can be expressed by the concentration terms such as…
Q: Analysis of Percent Organic Matter of * a soil sample was conducted and the data are shown below.…
A:
Q: There are 1,000 mg of a drug in 150ml. Find the percentage strength.
A: The strength of solutions is an important property and it is basically an application of percentage.…
Q: Determine the precision and accuracy of these data for dioxin. Sample 1 Sample 2 Sample 3 Known…
A:
Q: Five blood samples got these results: 0.752, 0.756, 0.752, 0.751, 0.760 ppm The standard deviation…
A:
Q: I The difference in standard deviations for the two instrumerntal methods is significant. II The…
A: F-test formula helps to perform statistical test. This test helps in finding that whether the two…
Q: In analytical chemistry, when and why do we do representative sampling?
A: Representative sample is defined as the small portion of the overall sample to determine the…
Q: All of the following are classified as an instrumental method of analysis EXCEPT: A.…
A: The analysis in which we rely on the instrument is called as instrumental method of analysis.
Q: What type of elution uses different proportions of solvent during separation process?
A: Elution is a process of separating one from a mixture by using eluent or solvent. By solvent…
Q: 7. Draw the structure of ethyl acetate and propyl acetate. Which substance is 6 expected to have the…
A: Introduction: Chromatography is a technique used to separate the mixture of solution. There are two…
Q: Which statement is valid when the multiple extraction and single extraction is compared. The…
A: Single extraction is the extraction in which only single extraction is done hich is one step whereas…
Q: n paper chromatography,
A: Paper chromatography was discovered by Synge and Martin in the year 1943.
Q: Peak area proportional to mass or concentration of the individual components of the sample injected.…
A:
Q: Peak area proportional to mass or concentration of the individual components of the sample injected.…
A: The area under the peak is a function of that compound's concentration in the sample.
Q: . What is the difference between temporary and permanent hardness?
A: Difference between temporary and permanent hardness :
Q: What is the retention factor (Rf) of the most polar and least polar spot present in the standard…
A: We have to give the retention factor (Rf) of the most polar and least polar spots present in the…
Q: Among the requirements for a substance to be a primary standard is low hygroscopicity. A) True False
A: True. Primary Standard should not absorb moisture from air. A substance that absorb or adsorb water…
Q: Mean [KIO3] (based on titration) = 0.02451 mol L-1 Standard deviation in the mean [KIO3] mol L-1…
A: [KIO3] mol L-1 (χi - μ) (χi - μ)2 0.02459 0.00008 6.4 * 10-9 0.02462 0.00011 1.21 * 10-8…
Q: Table 2. Data on Sample Analysis Volume of water sample (mL) : 10.00mL Trial Volume of EDTA (mL) ppm…
A:
Q: standard deviation for each treatment.
A: To determine the mean and standard deviation for each treatment. The mean is the sum of all the…
Q: Suggest a best method of separating the given mixtures: Assignment: answer with complete sentence…
A: In a separation method we can convert a mixture or solution into two or more distinct products .The…
Q: Use the table from ion exchange column to answer the following questions: Volume (mL) of Water…
A: Ca2+ + EDTA = Ca -EDTA + 2H+ thus 1 mol of EDTA reacts with 1 mol of Ca2+ ion Thus 1000 ml 1 (M)…
Q: please find the percent error not for each one but for the three trials together:)
A: In the given question we have to calculate the percent error for the density of water. we know…
Q: ample tub Deprot. samples Glucose Standards A B E #1 #2 O Standard/samples (r) 0.2 0.2…
A: The question involves the beer lambert law equation: A=ε c lA is the absorbance; c= concentrationl =…
Q: Retention time is the time taken for the mobile phase to reach the detector. True False
A: Answer - The above statement is false Explanation - Retention time - Retention time is the sum of…
Q: Categorize each as a determinate or an indeterminate error and further classify determinate errors…
A: A question based on tools in analytical chemistry that is to be accomplished.
Q: Describe the similarities and differences of the Bradford and Biuret method
A: The Biuret method is based on the reaction Cu2+ with functional groups in the protein’s peptide…
Q: Calculate the standard deviation of the following results: 20,19,18,10,15,14
A:
Q: How do you weigh hydgroscopic samples?
A: Hydgroscopic samples contain moisture. Therefore it is measured in digital analytical balance.
Q: Preparation of 50 g of a 10.0 % salt solution Find the reference value of a 10% salt solution and…
A: Concentration or Molarity is defined as “the mole of the solute per unit volume of the solution”. It…
Calculate the average and standard deviations for each solution (and solvent).
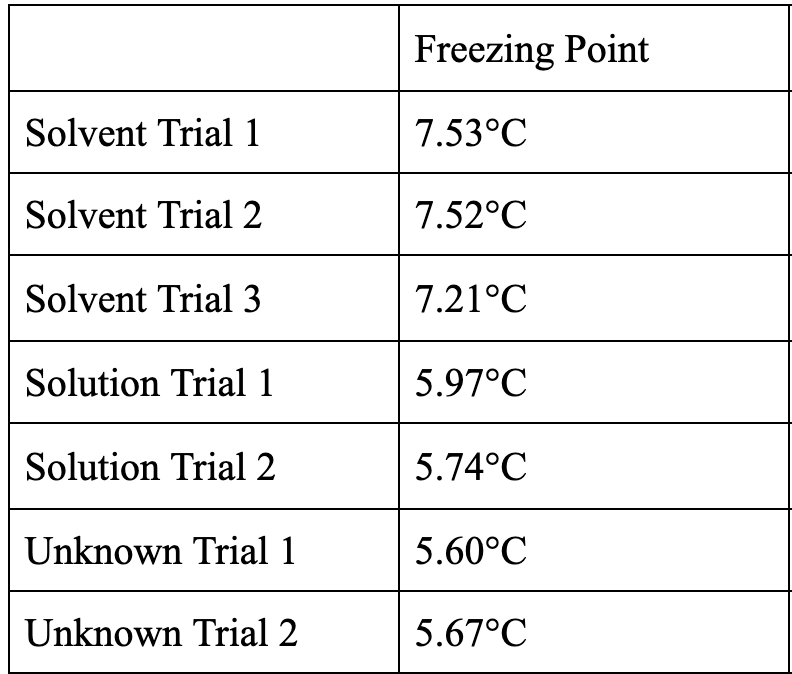

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

- How to prepare the solutions listed below Solution 1) 25 mL 7 M sulfuric acid and the respective dilution of this solution to prepare 50 mL 2 M sulfuric acid in deionised water. Sulfuric acid Mw = 98.079 g mol-1 Sulfuric acid density = 1.84 g mL-1 Sulfuric acid purity = 98% Solution 2) 100 mL 0.02M potassium permanganate potassium permanganate Mw = 158.03 g mol-1 potassium permanganate purity = 99%Melting point: 122-123 experimental product melting point: 120 mass of starting material: 1.04 mass of product: 0.85g what is limiting reagent and percent yield?The following reaction is carried out with [PCl5]i = 0.400 M at 25 °C. PCl5(g) ⇌ PCl3(g) + Cl2(g) K=0.00105 Use the small x approximation and “test x”. Report the percent (to two decimal places) when testing x.
- Using the percent purity calculations, determine the percent yield of synthesis of aspirin. Part I Synthesis of Aspirin Mass of salicylic acid used (g) 2.029g Volume of acetic anhydride used (mL) 5ml Mass of acetic anhydride used (vol. × 1.08 g/mL) 5.4g Mass of aspirin synthesized (g) 3.256g Part II Melting Temperature Data Melting temperature (°C) 133°C Part III Salicylic Acid Standard Stock Solution Initial mass of salicylic acid (g) 0.210g Moles of salicylic acid (mol) 0.0147 mol Initial molarity of salicylic acid (M) 0.724 M Part III Beer’s Law Data for Salicylic Acid Standard Solutions Trial Concentration (M) Absorbance Water (mL) 1 10 0.301 0 2 7.5 0.219 2.5 3 5.0 0.163 5.0 4 2.5 0.074 7.5 Best-fit line equation for the salicylic acid standards Test of the Purity of the Synthesized Aspirin Initial mass of aliquot of product (g)…1 Report on how to decrease the droplet evaporation time for liquidfuel combustion.Calculate the Constant Weight (in grams) of the Empty Crucibles. Show your solution. Crucible No. 1 2 3 Weighing 1 22.6035 22.0223 24.1535 Weighing 2 22.6017 22.0204 24.1533 Weighing 3 22.5994 22.0199 - Weighing 4 22.5992 - - Constant Weight, g ??? ??? ???
- Use E5C.1(a) to Plot the data in excel. Place two data sets on the same graph: one for temp vs. x and one for temp vs y (remember, x and y are the mole fractions and they belong on the x-axis). Don’t forget to include the boiling points of the pure substances – they are data, too. Add a trendline (polynomial, 3rd order should work) to each of the coexistence lines separately. Scale the plot appropriately and use the graph to answer the questions. Then complete E5C.1(b)Concentration of AR Stock Solution (ppmppm) 21.22 Unrounded Rounded Concentration of AR Stock Solution (μMμM) 42.7461 42.7What is ΔSsurr for a reaction at 28.6 °C with ΔHsys = 38.9 kJ mol-1 ? Express your answer in J mol-1 K-1 to at least two significant figures.
- Detailed calculations on how to prepare the solutions listed below Solution: 50 mL 1 M oxalic acid Oxalic acid Mw = 90.03 g mol-1 Oxalic acid purity = 98% Solution: 10 mL 3 M sulfuric acid Sulfuric acid Mw = 98.079 g mol-1 Sulfuric acid density = 1.84 g mL-1 Sulfuric acid purity = 98% Solution: 10 mL saturated potassium oxalate potassium oxalate (monohydrate used) Mw = 184.23 g mol-1 Solubility of potassium oxalate in water at 25°C = 360 mg mL-1 Solution :20 mL 3% hydrogen peroxide Hydrogen peroxide is commercially available as a 32% solution.DATA Note: Use the videos as references except when data is provided here. Table 1. Selection of Recrystallizing Solvent. Solvents Dissolution at room temperature? (+ or −) Dissolution at elevated temperature? (+ or −) Distilled water - - Acetone + n/a 95% ethanol - + Toluene + n/a Solid sample: Naphthalene Table 2. Mass Measurements. Mass, g Sample solid 0.5021 Empty watch glass 35.7602 Watch glass with recrystallized sample 36.2485 Table 3. Melting Point Determination. Temperature, oC Appearance of Sample in Capillary Tube … Solid 78.4 Solid 79.3 Solid with signs of liquid 79.9 Solid, liquid 80.6 Liquid, solid 81.3 Liquid with signs of solid … Liquid Theoretical Melting Point, oC: 80.2°C (176.4°F) QUESTIONS Which solvent should be used as the recrystallizing solvent? Check if your chosen solvent satisfies all characteristics of a good…100.0 cm3 of a 1.234 mol.dm-3 solution of nickel(II) nitrate was added to 150.0 cm3 of a 1.178 mol.dm-3 solution of sodium carbonate. Determine the limiting reagent

