Mid-Pacific Science 3. Create an isotope of platinum (atomic #78) that undergoes alpha (a) decay. a. What changes occur to the isotope as it decays? symbol proton amount neutron amount Before Decay After Decay mass charge (atomic number) Summarize these changes with your partner. b. What is the mass number and charge of an alpha particle? What is an alpha particle made up of? He c. Construct a nuclear equation to represent the a decay of the platinum isotope you created. Pt -> + He d. Use your answer to part c to mathematically support the statements below: In a nuclear reaction, the sum of the mass numbers of the reactants equals the sum of the mass numbers of the products. In a nuclear reaction, the total charge of the reactants equals the total charge of the products. 4. Create an isotope of fluorine that undergoes beta minus (ẞ-) decay. a. What changes occur to the isotope as it decays? Topic 7 Atomic Nuclear Particle Physics page #3
Mid-Pacific Science 3. Create an isotope of platinum (atomic #78) that undergoes alpha (a) decay. a. What changes occur to the isotope as it decays? symbol proton amount neutron amount Before Decay After Decay mass charge (atomic number) Summarize these changes with your partner. b. What is the mass number and charge of an alpha particle? What is an alpha particle made up of? He c. Construct a nuclear equation to represent the a decay of the platinum isotope you created. Pt -> + He d. Use your answer to part c to mathematically support the statements below: In a nuclear reaction, the sum of the mass numbers of the reactants equals the sum of the mass numbers of the products. In a nuclear reaction, the total charge of the reactants equals the total charge of the products. 4. Create an isotope of fluorine that undergoes beta minus (ẞ-) decay. a. What changes occur to the isotope as it decays? Topic 7 Atomic Nuclear Particle Physics page #3
Chapter10: Atomic Physics
Section: Chapter Questions
Problem 42Q
Related questions
Question
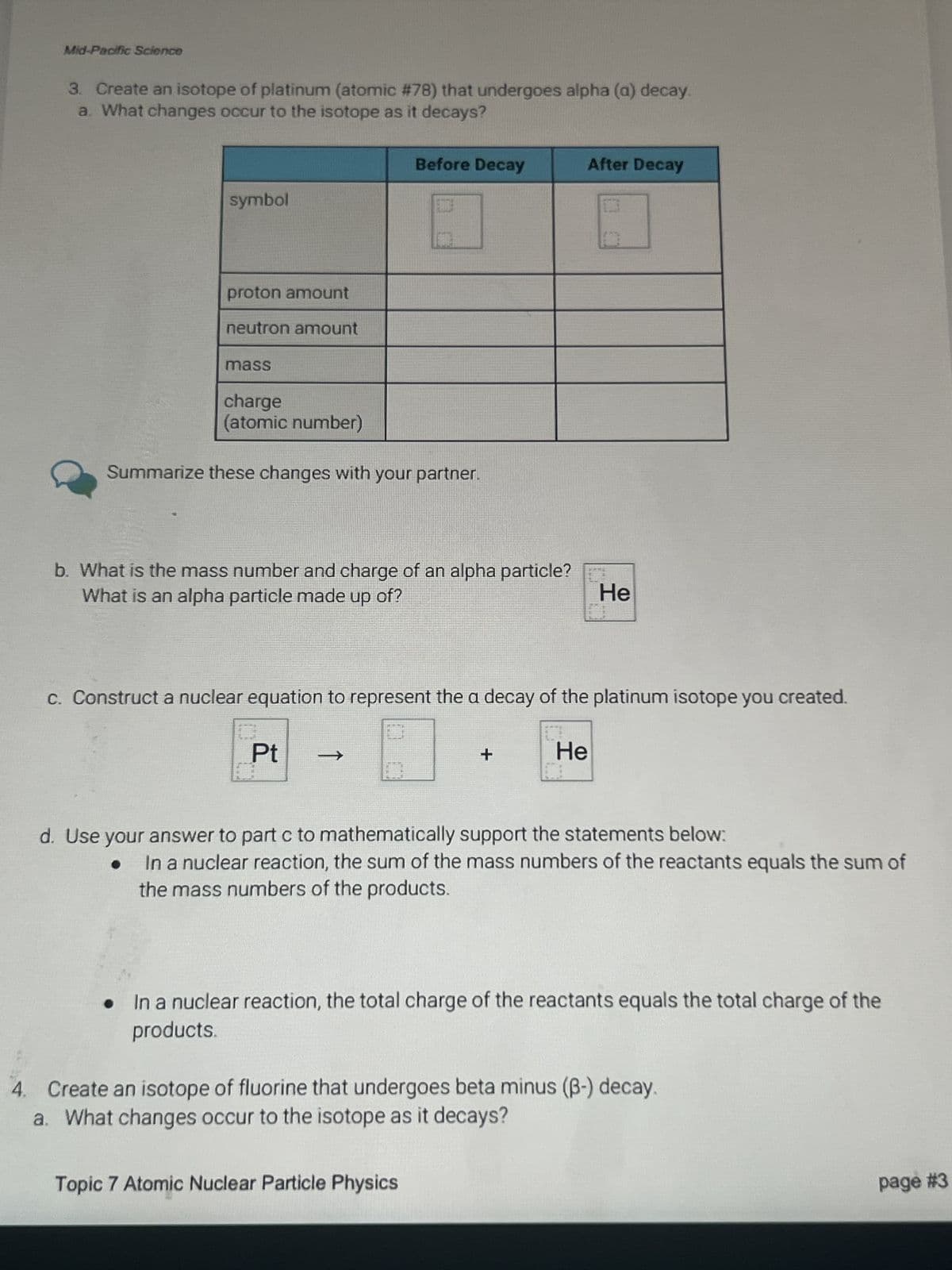
Transcribed Image Text:Mid-Pacific Science
3. Create an isotope of platinum (atomic #78) that undergoes alpha (a) decay.
a. What changes occur to the isotope as it decays?
symbol
proton amount
neutron amount
Before Decay
After Decay
mass
charge
(atomic number)
Summarize these changes with your partner.
b. What is the mass number and charge of an alpha particle?
What is an alpha particle made up of?
He
c. Construct a nuclear equation to represent the a decay of the platinum isotope you created.
Pt
->
+
He
d. Use your answer to part c to mathematically support the statements below:
In a nuclear reaction, the sum of the mass numbers of the reactants equals the sum of
the mass numbers of the products.
In a nuclear reaction, the total charge of the reactants equals the total charge of the
products.
4. Create an isotope of fluorine that undergoes beta minus (ẞ-) decay.
a. What changes occur to the isotope as it decays?
Topic 7 Atomic Nuclear Particle Physics
page #3
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you


Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

Astronomy
Physics
ISBN:
9781938168284
Author:
Andrew Fraknoi; David Morrison; Sidney C. Wolff
Publisher:
OpenStax