MISSED THIS? Read Section 18.2 (Pages 788 - 799) ; Watch KCV 18.2B, IWES 18.2, 18.3. A 100.0-mL buffer solution is 0.175 M in HC1O and 0.150 M in NaClO. ry.com/myct/itemView?assignmentProblemID=D143793616&offset%3Dprev CHE154-H Gen Chem II Bronikowski S20 ck and Hints );Watch 10.150 M Part A What is the initial pH of this solution? Express your answer to two decimal places. ΑΣΦ pH = %3D
MISSED THIS? Read Section 18.2 (Pages 788 - 799) ; Watch KCV 18.2B, IWES 18.2, 18.3. A 100.0-mL buffer solution is 0.175 M in HC1O and 0.150 M in NaClO. ry.com/myct/itemView?assignmentProblemID=D143793616&offset%3Dprev CHE154-H Gen Chem II Bronikowski S20 ck and Hints );Watch 10.150 M Part A What is the initial pH of this solution? Express your answer to two decimal places. ΑΣΦ pH = %3D
Chapter14: Principles Of Neutralization Titrations
Section: Chapter Questions
Problem 14.3QAP
Related questions
Question
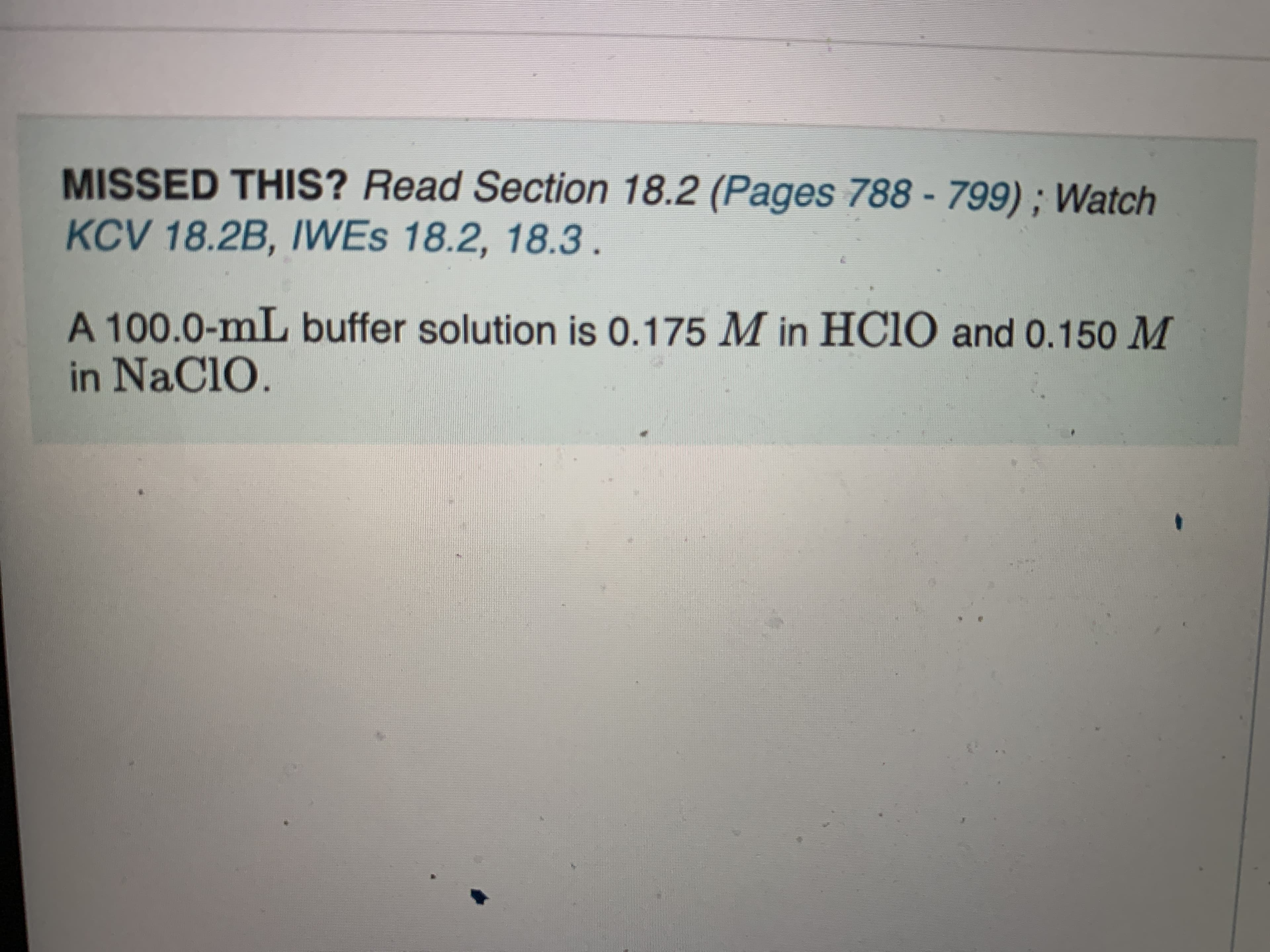
Transcribed Image Text:MISSED THIS? Read Section 18.2 (Pages 788 - 799) ; Watch
KCV 18.2B, IWES 18.2, 18.3.
A 100.0-mL buffer solution is 0.175 M in HC1O and 0.150 M
in NaClO.
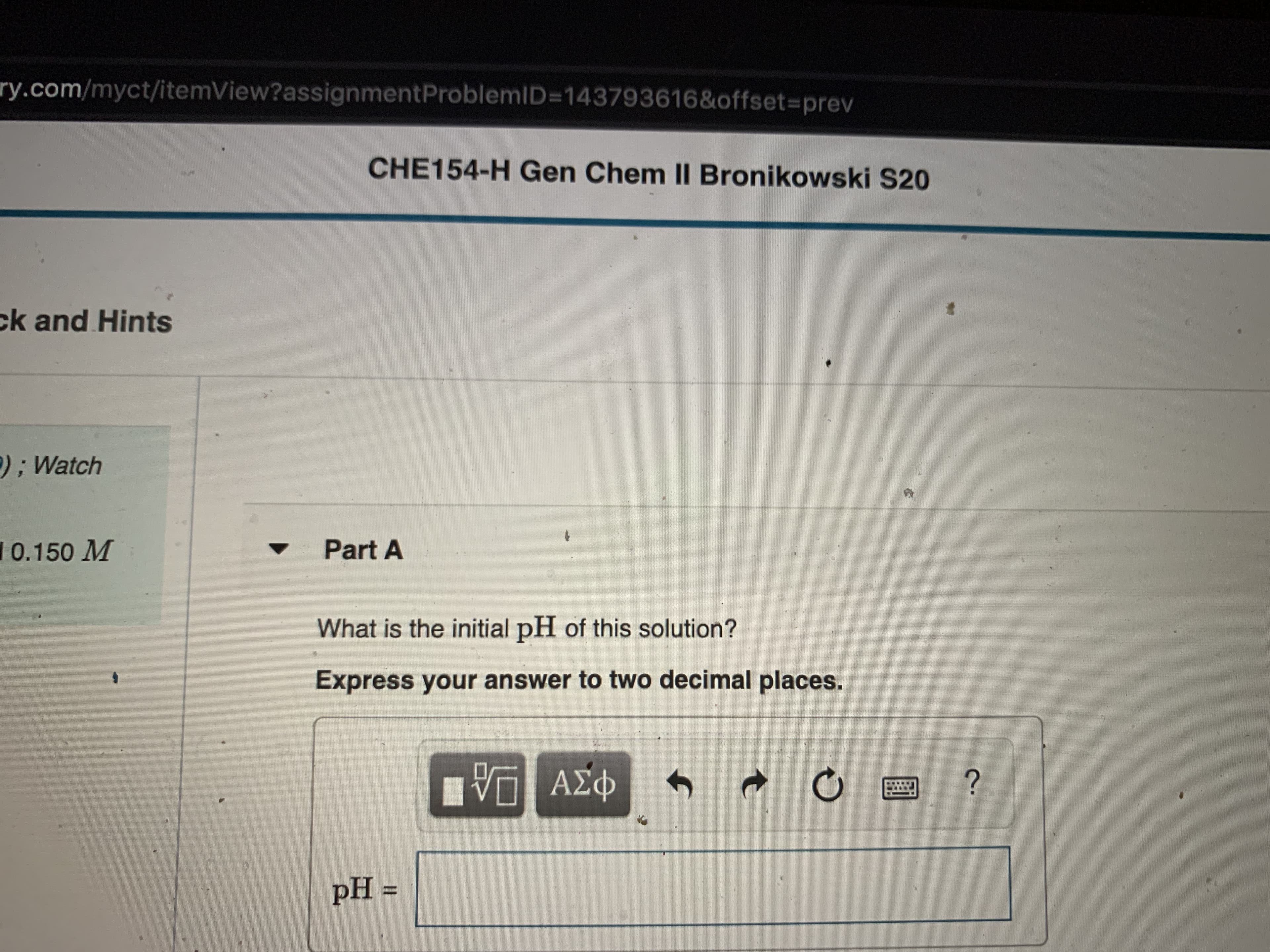
Transcribed Image Text:ry.com/myct/itemView?assignmentProblemID=D143793616&offset%3Dprev
CHE154-H Gen Chem II Bronikowski S20
ck and Hints
);Watch
10.150 M
Part A
What is the initial pH of this solution?
Express your answer to two decimal places.
ΑΣΦ
pH =
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you



Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning



Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole