Model 1: Structures and boiling points of selected organic molecules Nitrogen bonding options: ཡིན་ན་ H amine amide H Functional Molecular Boiling Example Structure Weight Point Group H₂ H₂ Alkane 72 36°C H3C CH3 CH3 Amine (A) CH3 73 36°C H3C H2 H3C CH3 Amine (B) 73 56°C H₂ H2 NH₂ Amine (C) H3C CH 73 63°C -CH3 H₂ Amine (D) H3C Alcohol H3C 73 78°C NH2 EU H₂ H₂ 74 OH 118°C Carboxylic Acid H3C 74 141°C OH Amide H3C n=o 73 218°C NH₂
Model 1: Structures and boiling points of selected organic molecules Nitrogen bonding options: ཡིན་ན་ H amine amide H Functional Molecular Boiling Example Structure Weight Point Group H₂ H₂ Alkane 72 36°C H3C CH3 CH3 Amine (A) CH3 73 36°C H3C H2 H3C CH3 Amine (B) 73 56°C H₂ H2 NH₂ Amine (C) H3C CH 73 63°C -CH3 H₂ Amine (D) H3C Alcohol H3C 73 78°C NH2 EU H₂ H₂ 74 OH 118°C Carboxylic Acid H3C 74 141°C OH Amide H3C n=o 73 218°C NH₂
Biology (MindTap Course List)
11th Edition
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Chapter3: The Chemistry Of Life: Organic Compounds
Section: Chapter Questions
Problem 2TYU: VISUALIZE The structures depicted are (a) enantiomers (b) different views of the same molecule (c)...
Related questions
Question
- Label each Amine (A–D) in Table 1 as primary, secondary, or tertiary.
- Which classes of
amines – primary, secondary, or tertiary – can participate in hydrogen bonding with other identical molecules? Discuss as a team, and record your consensus. - How many different combinations of hydrogen bonds are possible between the following two molecules? redraw the molecules, showing a hydrogen bond between them.
- Compare Amines A–D in Model 1. Discuss as a team, and explain why three of the amines have a higher boiling point than the
alkane . - One of the amines has the same boiling point as the alkane. Explain.
- Compare Amine (A) and Amine (B). Explain the difference in boiling point.
- Compare Amine (B) with amine (C). Explain the difference in boiling point.
- Compare Amine (C) with amine (D). Explain the difference in boiling point.
- Compare Amine (D) with the alcohol. Work as a team to explain the difference in boiling point.
- Compare the amide with the alcohol. Explain the difference in boiling point.
- Would you expect amines and amides with short carbon chains to be soluble in water? Work as a team to explain your answer. (Hint: Recall the solubility of carboxylic acids discussed in CA 39.)
- Which class of amine has the highest boiling point? Why?
- What was one strength of your team today? Why was this a strength?
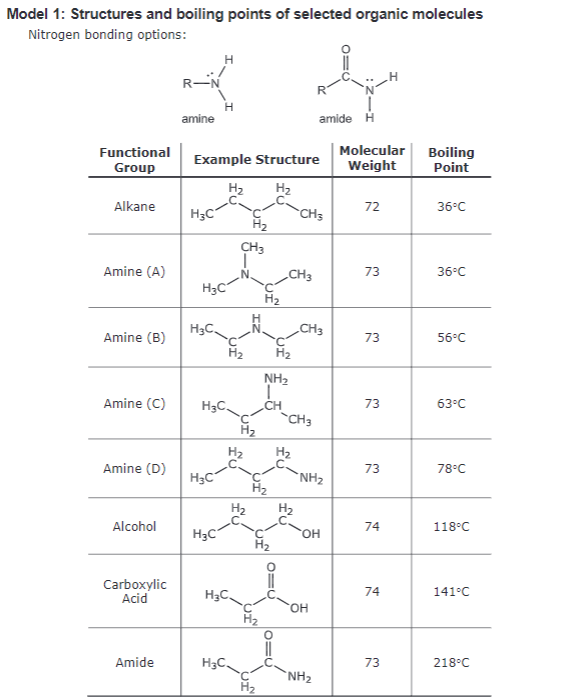
Transcribed Image Text:Model 1: Structures and boiling points of selected organic molecules
Nitrogen bonding options:
ཡིན་ན་
H
amine
amide H
Functional
Molecular
Boiling
Example Structure
Weight
Point
Group
H₂
H₂
Alkane
72
36°C
H3C
CH3
CH3
Amine (A)
CH3
73
36°C
H3C
H2
H3C
CH3
Amine (B)
73
56°C
H₂
H2
NH₂
Amine (C)
H3C
CH
73
63°C
-CH3
H₂
Amine (D)
H3C
Alcohol
H3C
73
78°C
NH2
EU
H₂
H₂
74
OH
118°C
Carboxylic
Acid
H3C
74
141°C
OH
Amide
H3C
n=o
73
218°C
NH₂
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 1 steps with 1 images

Recommended textbooks for you

Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax


Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax


Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College