Consider the given data (Solubility Tests, Solubility Group, Chemical Tests, and Functional Group) in the experiment. Based on the results of the solubility tests and chemical tests, what is the most probable structure of C4H6O2? Draw your answer. You may draw the compound using line-bond formula
Consider the given data (Solubility Tests, Solubility Group, Chemical Tests, and Functional Group) in the experiment. Based on the results of the solubility tests and chemical tests, what is the most probable structure of C4H6O2? Draw your answer. You may draw the compound using line-bond formula
Biomedical Instrumentation Systems
1st Edition
ISBN:9781133478294
Author:Chatterjee
Publisher:Chatterjee
Chapter19: Biomedical Laboratory Instrumentation
Section: Chapter Questions
Problem 7P
Related questions
Question
Consider the given data (Solubility Tests, Solubility Group, Chemical Tests, and Functional Group) in the experiment.
- Based on the results of the solubility tests and chemical tests, what is the most probable structure of C4H6O2? Draw your answer. You may draw the compound using line-bond formula OR Lewis structure.
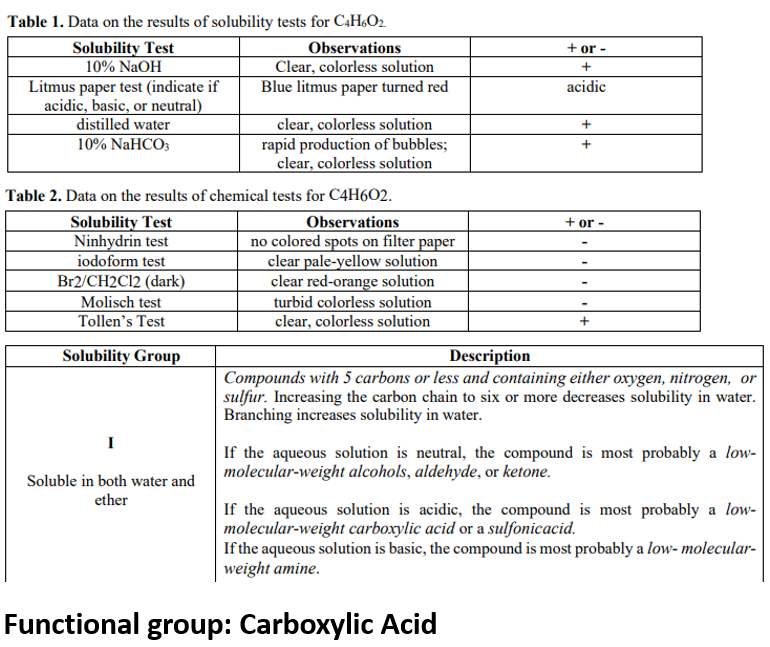
Transcribed Image Text:Table 1. Data on the results of solubility tests for C4H&O2
Observations
Clear, colorless solution
Blue litmus paper turned red
Solubility Test
10% NaOH
Litmus paper test (indicate if
acidic, basic, or neutral)
distilled water
10% NaHCO;
+ or -
acidic
clear, colorless solution
rapid production of bubbles;
clear, colorless solution
+
Table 2. Data on the results of chemical tests for C4H602.
Solubility Test
Ninhydrin test
iodoform test
Br2/CH2C12 (dark)
Molisch test
Tollen's Test
+ or -
Observations
no colored spots on filter paper
clear pale-yellow solution
clear red-orange solution
turbid colorless solution
clear, colorless solution
Solubility Group
Description
Compounds with 5 carbons or less and containing either oxygen, nitrogen, or
sulfur. Increasing the carbon chain to six or more decreases solubility in water.
Branching increases solubility in water.
I
If the aqueous solution is neutral, the compound is most probably a low-
molecular-weight alcohols, aldehyde, or ketone.
Soluble in both water and
ether
If the aqueous solution is acidic, the compound is most probably a low-
molecular-weight carboxylic acid or a sulfonicacid.
If the aqueous solution is basic, the compound is most probably a low- molecular-
weight amine.
Functional group: Carboxylic Acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you



