Molecular formula Lewis Structure SO3 CO2 NO2 Total number ofe groups around the central atom Number of lone pairs around the central atom Electron geometry Molecular geometry Approximate bond angles Polarity
Molecular formula Lewis Structure SO3 CO2 NO2 Total number ofe groups around the central atom Number of lone pairs around the central atom Electron geometry Molecular geometry Approximate bond angles Polarity
Chapter9: Covalent Bonding: Orbitals
Section: Chapter Questions
Problem 87CP: Consider the following computer-generated model of caffeine: Complete a Lewis structure for caffeine...
Related questions
Question
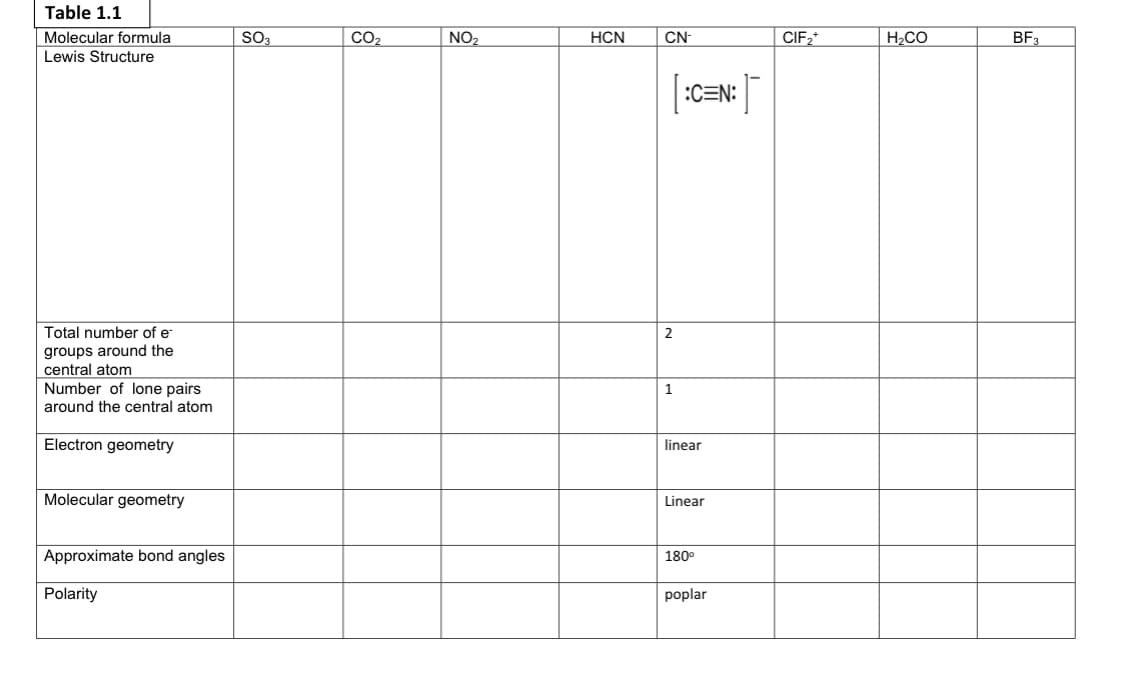
Transcribed Image Text:Table 1.1
Molecular formula
SO3
CO2
NO2
HCN
CN-
CIF2*
H2CO
BF3
Lewis Structure
:C=N:
Total number of e
2
groups around the
central atom
Number of lone pairs
around the central atom
1
Electron geometry
linear
Molecular geometry
Linear
Approximate bond angles
180°
Polarity
poplar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning