Moles and Chemical Formulas Finding the Simplest Formula 1. Mass of empty crucible + cover 2. Initial appearance of the magnesium 3. Mass of crucible + cover + magnesium 4. Mass of crucible + cover + oxide product Calculations 5. Mass of magnesium 6. Mass of magnesium compound 7. Mass of oxygen in the product 8. Moles of Mg (Show calculations.) 9. Moles of O (Show calculations.) 10. Which number of moles (Mg or O) is smaller 38.2337 Silver color 38.4285 38.5578 Exp3 OD OD OD 6.0 OD g g mole mole
Moles and Chemical Formulas Finding the Simplest Formula 1. Mass of empty crucible + cover 2. Initial appearance of the magnesium 3. Mass of crucible + cover + magnesium 4. Mass of crucible + cover + oxide product Calculations 5. Mass of magnesium 6. Mass of magnesium compound 7. Mass of oxygen in the product 8. Moles of Mg (Show calculations.) 9. Moles of O (Show calculations.) 10. Which number of moles (Mg or O) is smaller 38.2337 Silver color 38.4285 38.5578 Exp3 OD OD OD 6.0 OD g g mole mole
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 20CR: Solid calcium carbide (CaC2)reacts with liquid water to produce acetylene gas (C2H2)and aqueous...
Related questions
Question
Transcribed Image Text:기
1:01
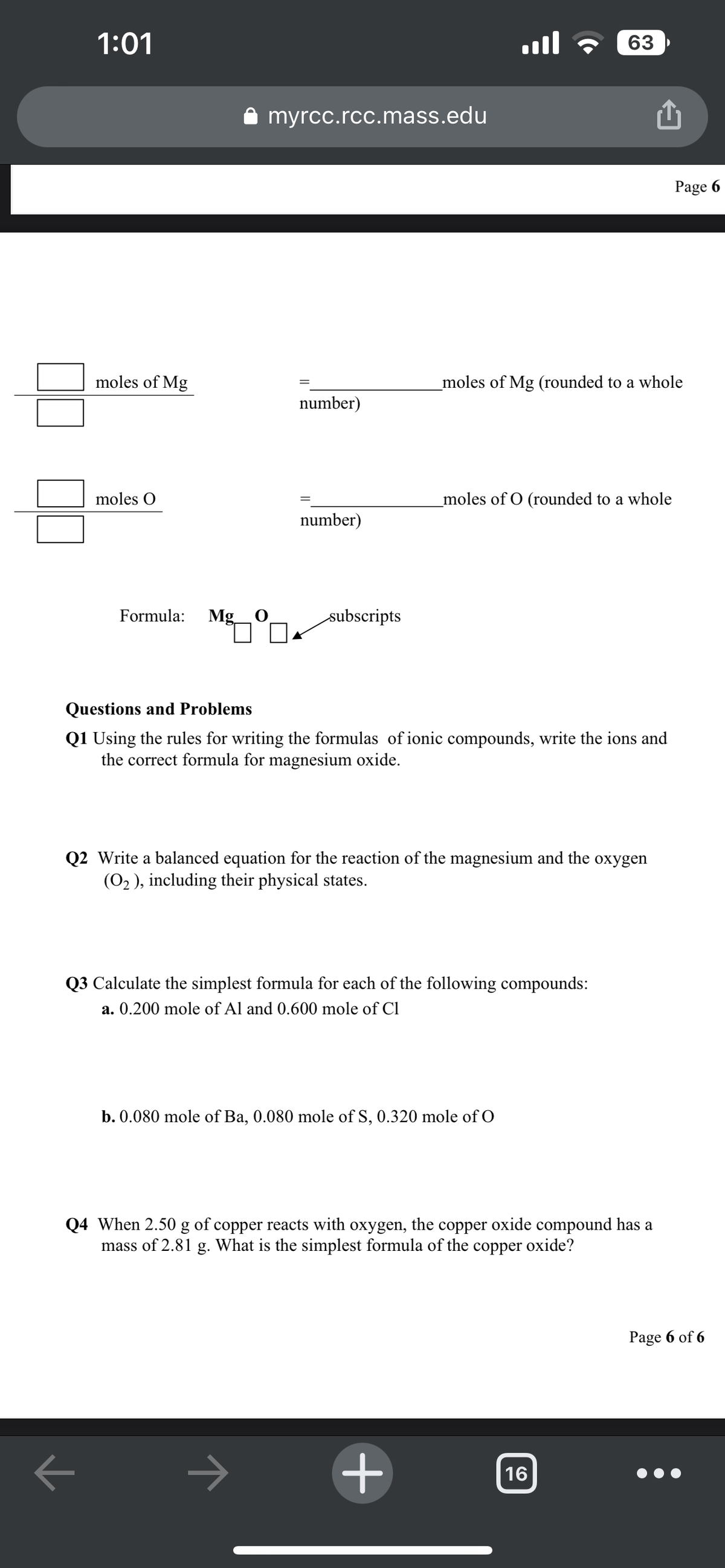
moles of Mg
moles O
Formula: Mg O
myrcc.rcc.mass.edu
=
number)
=
number)
→
subscripts
ا...
Questions and Problems
Q1 Using the rules for writing the formulas of ionic compounds, write the ions and
the correct formula for magnesium oxide.
_moles of Mg (rounded to a whole
_moles of O (rounded to a whole
Q2 Write a balanced equation for the reaction of the magnesium and the oxygen
(O₂), including their physical states.
Q3 Calculate the simplest formula for each of the following compounds:
a. 0.200 mole of Al and 0.600 mole of Cl
b. 0.080 mole of Ba, 0.080 mole of S, 0.320 mole of O
63
+
Q4 When 2.50 g of copper reacts with oxygen, the copper oxide compound has a
mass of 2.81 g. What is the simplest formula of the copper oxide?
16
Page 6
Page 6 of 6

Transcribed Image Text:1:01
1 of 2
Finding the Simplest Formula
Moles and Chemical Formulas
기
✰ myrcc.rcc.mass.edu
1. Mass of empty crucible + cover
2. Initial appearance of the magnesium
3. Mass of crucible + cover + magnesium
4. Mass of crucible + cover + oxide product
Calculations
5. Mass of magnesium
6. Mass of magnesium compound
7. Mass of oxygen in the product
8. Moles of Mg
(Show calculations.)
9. Moles of O
REPORT SHEET
(Show calculations.)
10. Which number of moles (Mg or O) is smaller
+
38.2337
Silver color
38.4285
38.5578
ا...
16
LAB
Exp3
63
OD OD
g
OD
OD
6.0
g
g
g
mole
mole
Page
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning