Most acidic Least acidic a Part 3 out of 3 This ranking is explained by: Electronegativity makes the alcohol OH most acidic; the conjugate base of the a-CH has negatively charged C, and so is least acidic. Electronegativity and resonance makes the COOH most acidic; the conjugate base of the alcohol OH has no stabilization, and so is least acidic. Resonance makes the a-CH most acidic; the conjugate base of the CH, has no stabilization, and so is least acidic. Electronegativity and resonance makes the COOH most acidic; the conjugate base of the CH, has no stabilization, and so is least acidic.
Most acidic Least acidic a Part 3 out of 3 This ranking is explained by: Electronegativity makes the alcohol OH most acidic; the conjugate base of the a-CH has negatively charged C, and so is least acidic. Electronegativity and resonance makes the COOH most acidic; the conjugate base of the alcohol OH has no stabilization, and so is least acidic. Resonance makes the a-CH most acidic; the conjugate base of the CH, has no stabilization, and so is least acidic. Electronegativity and resonance makes the COOH most acidic; the conjugate base of the CH, has no stabilization, and so is least acidic.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter14: Acid-base Equilibria
Section: Chapter Questions
Problem 33E: Gastric juice, the digestive ?uid produced in the stomach, contains hydrochloric acid, HCl, Milk of...
Related questions
Question
Part 3
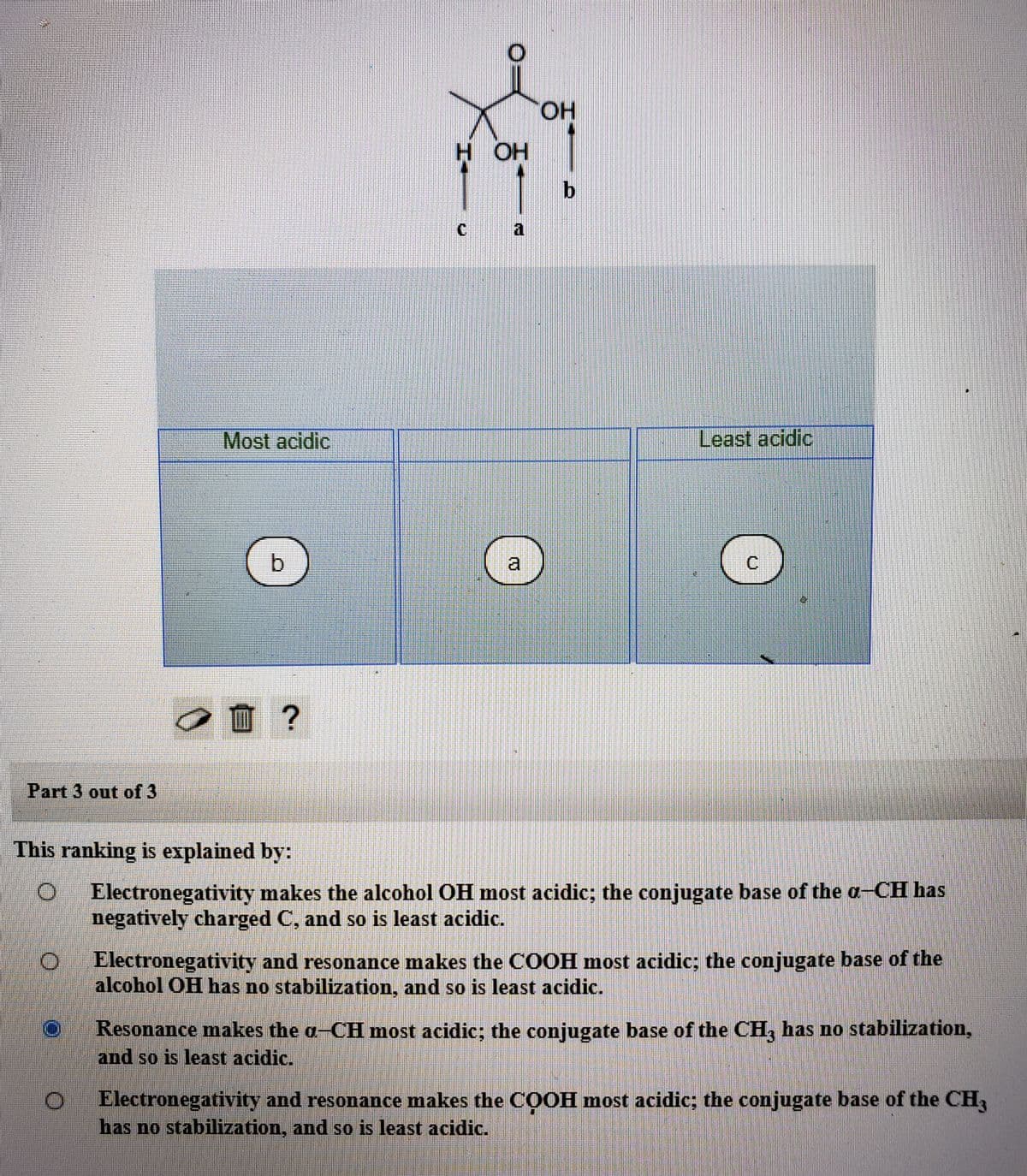
Transcribed Image Text:HO.
H OH
C a
Most acidic
Least acidic
Part 3 out of 3
This ranking is explained by:
Electronegativity makes the alcohol OH most acidic; the conjugate base of the a-CH has
negatively charged C, and so is least acidic.
Electronegativity and resonance makes the COOH most acidic; the conjugate base of the
alcohol OH has no stabilization, and so is least acidic.
Resonance makes the a-CH most acidic; the conjugate base of the CH, has no stabilization,
and so is least acidic.
Electronegativity and resonance makes the COOH most acidic; the conjugate base of the CH,
has no stabilization, and so is least acidic.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning