MReview I Contants Perodic Table candard entroples for selected substances at 25 C Part A Substance S" (J/(mol -K)) Oy(g) SO (8) 205.2 Calculate AS for the balanced chemical equation 248.2 H,S(x) + 20, (R) - H,0(g) + SO, (R) So, (R) 256.8 HO(g) Express the entropy change to four significant figures and include the appropriate units. 188.8 H2S(x) 205.8 AS 170.65 S Submit Prexisn Ats Reauest Anser X Incorrect; Try Again; 4 attempts remaining
MReview I Contants Perodic Table candard entroples for selected substances at 25 C Part A Substance S" (J/(mol -K)) Oy(g) SO (8) 205.2 Calculate AS for the balanced chemical equation 248.2 H,S(x) + 20, (R) - H,0(g) + SO, (R) So, (R) 256.8 HO(g) Express the entropy change to four significant figures and include the appropriate units. 188.8 H2S(x) 205.8 AS 170.65 S Submit Prexisn Ats Reauest Anser X Incorrect; Try Again; 4 attempts remaining
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter8: Electrochemistry And Ionic Solutions
Section: Chapter Questions
Problem 8.75E: Under what conditions does the extended Debye-Huckel law, equation 8.52, become the Debye-Hckel...
Related questions
Question
7
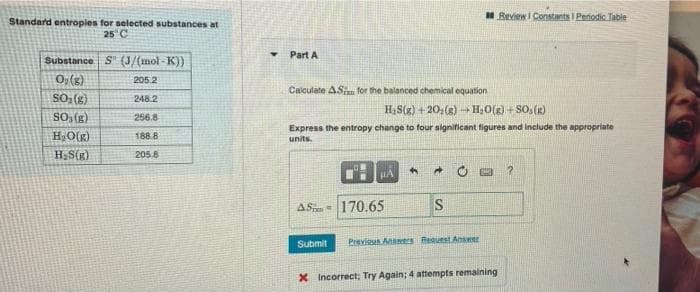
Transcribed Image Text:MReview I Constants I Periodic Table
Standard entropies for selected substances at
25 C
Part A
Substance S" (J/(mol - K))
0; (g)
SO; {g)
So, (g)
205.2
Calculate AS for the balanced chemical equation
248.2
H;S(x) + 20;(g) - H;0(g) + SO, (R)
256.8
HO(g)
Express the entropy change to four significant figures and inelude the appropriate
units.
188.8
H,S(x)
205.8
ASim= 170.65
IS
Submit
Previsu Aers Request Anser
X Incorrect: Try Again; 4 attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,