n the 1.0 M CuSO4 solution, the following species are present: Cu²+, SO4², H₂O, H³O+, and OH- . Based on the observed changes that occurred in each electrode, write the RHR, OHR, and the balanced overall reaction of the electrolytic cell. The reactions should be consistent with the observations.
n the 1.0 M CuSO4 solution, the following species are present: Cu²+, SO4², H₂O, H³O+, and OH- . Based on the observed changes that occurred in each electrode, write the RHR, OHR, and the balanced overall reaction of the electrolytic cell. The reactions should be consistent with the observations.
Chapter11: Dynamic Electrochemistry
Section: Chapter Questions
Problem 1P
Related questions
Question
In the 1.0 M CuSO4 solution, the following species are present: Cu²+, SO4², H₂O, H³O+, and OH- . Based on the observed changes that occurred in each electrode, write the RHR, OHR, and the balanced overall reaction of the
electrolytic cell. The reactions should be consistent with the observations.
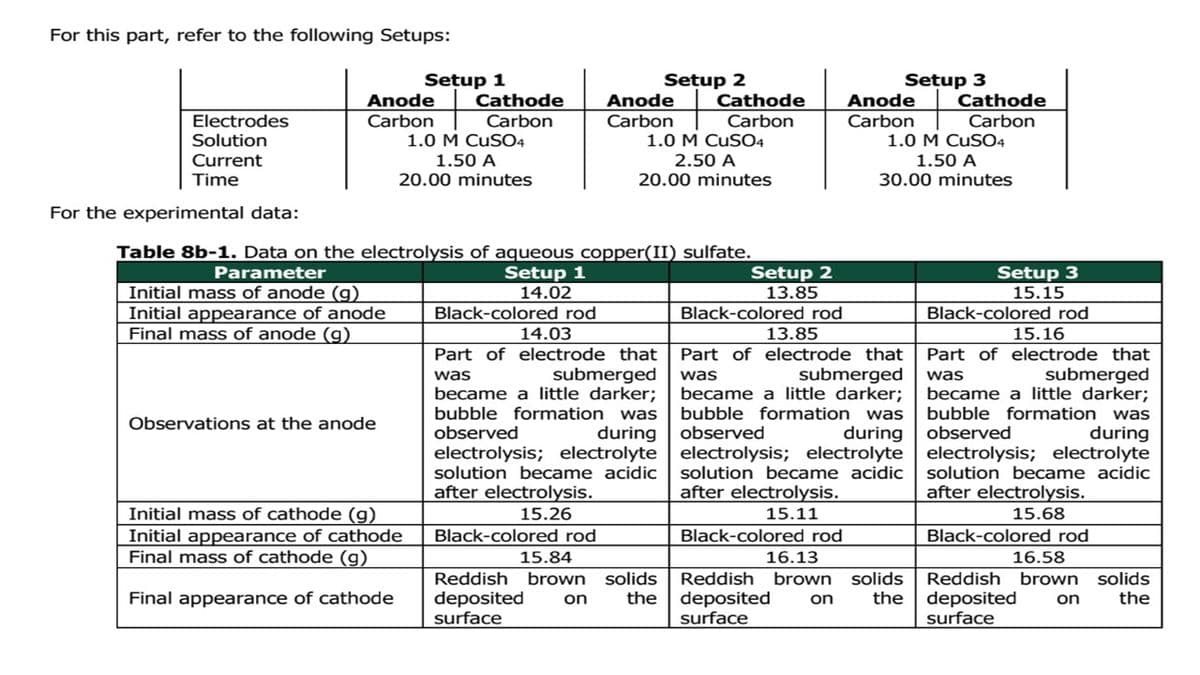
Transcribed Image Text:For this part, refer to the following Setups:
Electrodes
Solution
Current
Time
For the experimental data:
Anode
Carbon
Setup 1
Cathode
Carbon
1.0 M CUSO4
1.50 A
20.00 minutes
Initial mass of anode (g)
Initial appearance of anode
Final mass of anode (g)
Observations at the anode
Table 8b-1. Data on the electrolysis of aqueous copper(II) sulfate.
Parameter
Setup 1
14.02
Black-colored rod
14.03
Part of electrode that
was
submerged
became a little darker;
bubble formation was
observed
during
electrolysis; electrolyte
solution became acidic
after electrolysis.
15.26
Black-colored rod
Setup 2
Cathode
Carbon
1.0 M CUSO4
2.50 A
20.00 minutes
Initial mass of cathode (g)
Initial appearance of cathode
Final mass of cathode (g)
Final appearance of cathode
Anode
Carbon
15.84
Reddish brown solids
deposited on the
surface
Setup 2
13.85
Black-colored rod
Anode
Carbon
Setup 3
13.85
Part of electrode that
was
submerged
became a little darker;
bubble formation was
observed
during
electrolysis; electrolyte
solution became acidic
after electrolysis.
15.11
Black-colored rod
1.0 M CUSO4
1.50 A
30.00 minutes
16.13
Reddish brown solids
deposited on the
surface
Cathode
Carbon
Setup 3
15.15
Black-colored rod
15.16
Part of electrode that
was
submerged
became a little darker;
bubble formation was
observed
during
electrolysis; electrolyte
solution became acidic
after electrolysis.
15.68
Black-colored rod
16.58
Reddish brown solids
deposited on the
surface
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning