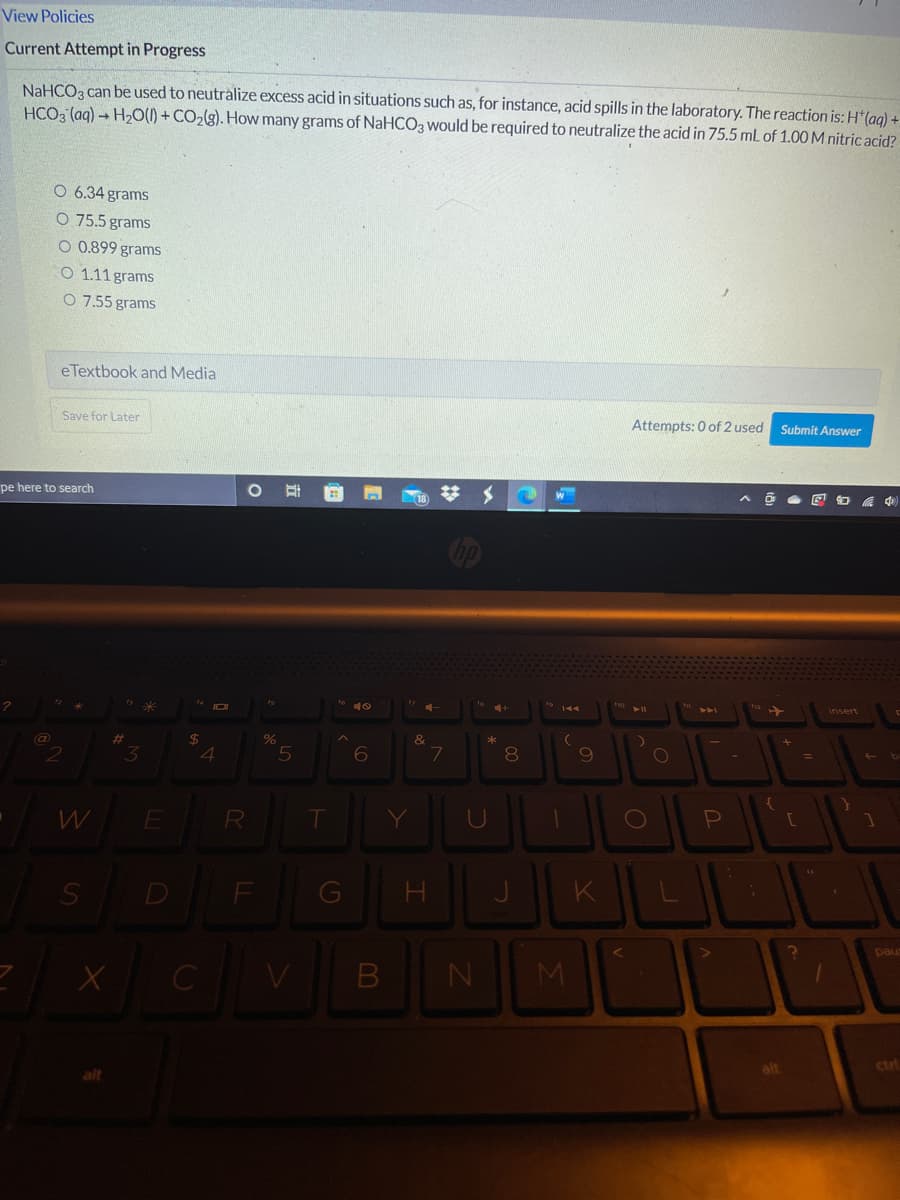
NaHCO, can be used to neutralize excess acid in situations such as, for instance, acid spills in the laboratory. The reaction is: H'(ag) = HCO3 (aq) H2O(1) + CO2(g). How many grams of NaHCO3 would be required to neutralize the acid in 75.5 mL of 1.00 M nitric acid? O 6.34 grams O 75.5 grams O 0.899 grams O 1.11 grams O 7.55 grams eTextbook and Media Attempts: 0 of 2 used Submit Answer Save for Later
NaHCO, can be used to neutralize excess acid in situations such as, for instance, acid spills in the laboratory. The reaction is: H'(ag) = HCO3 (aq) H2O(1) + CO2(g). How many grams of NaHCO3 would be required to neutralize the acid in 75.5 mL of 1.00 M nitric acid? O 6.34 grams O 75.5 grams O 0.899 grams O 1.11 grams O 7.55 grams eTextbook and Media Attempts: 0 of 2 used Submit Answer Save for Later
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 62QAP: Ten mL of concentrated H3PO4 (91.7% by mass, d=1.69g/mL) was accidentally poured into a beaker...
Related questions
Question
Transcribed Image Text:View Policies
Current Attempt in Progress
NaHCO3 can be used to neutralize excess acid in situations such as, for instance, acid spills in the laboratory. The reaction is: H*(ag) +
HCO3 (aq) → H20(1) + CO2(g). How many grams of NaHCO3 would be required to neutralize the acid in 75.5 mL of 1.00 M nitric acid?
O 6.34 grams
O 75.5 grams
O 0.899 grams
O 1.11 grams
O 7.55 grams
eTextbook and Media
Save for Later
Attempts: 0 of 2 used Submit Answer
pe here to search
18
Cip
insert
IOI
林
24
7
8
Y
D
G
K
pau
M
ctr
alt
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning