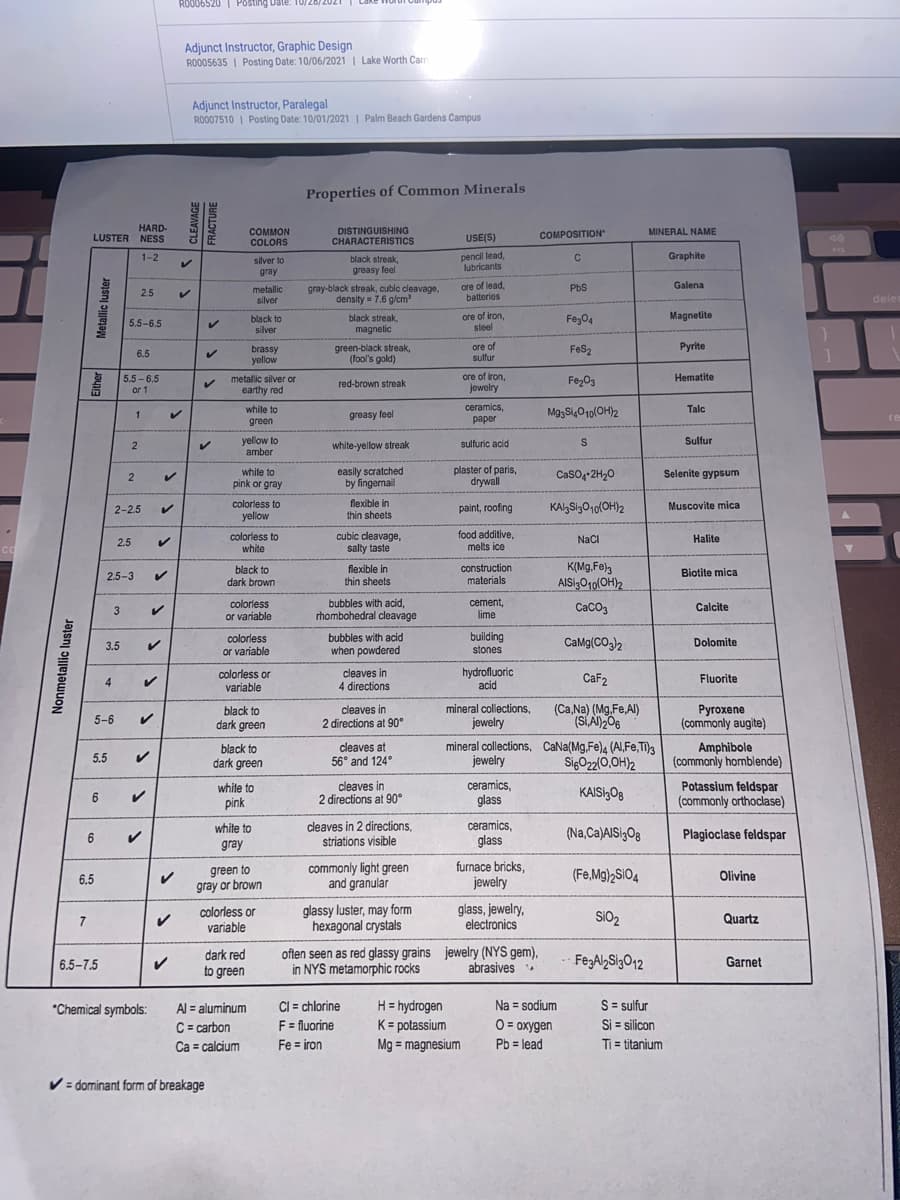
NAME: ESC-ASSIGNMENT #2 (CHAPTER 2 PART TWO): MINERAL IDENTIFICATION DIRECTIONS: For each of the following unidentified minerals, you are provided with a photo and some physical properties. Refer to the table of "Properties of Common Minerals" (next page) to determine the identity of each mineral described. Each correct answer is worth 2 points. DUE: January 19th 1. Nonmetallic Luster; Hardness: 3; Uses: Cement, Lime Color: colorless to white; Other: Reacts (bubbles) with acid Mineral Identity: 2. Luster: Either metallic or earthy; Hardness: 5.5 to 6.5, but the earthy variety can be as soft as 1.0-1.5 No Cleavage; Color: metallic silver or earthy red Mineral Identity: 3. Nonmetallic Luster; Hardness: 2.5-3; Cleavage: Thin sheet; Color: black to dark brown; Uses: Construction material Mineral Identity: 4. Nonmetallic Luster; Hardness: 2.5; Cleavage: cubic; Color: colorless to white; Uses: Food additive, Melts ice Mineral Identity:
NAME: ESC-ASSIGNMENT #2 (CHAPTER 2 PART TWO): MINERAL IDENTIFICATION DIRECTIONS: For each of the following unidentified minerals, you are provided with a photo and some physical properties. Refer to the table of "Properties of Common Minerals" (next page) to determine the identity of each mineral described. Each correct answer is worth 2 points. DUE: January 19th 1. Nonmetallic Luster; Hardness: 3; Uses: Cement, Lime Color: colorless to white; Other: Reacts (bubbles) with acid Mineral Identity: 2. Luster: Either metallic or earthy; Hardness: 5.5 to 6.5, but the earthy variety can be as soft as 1.0-1.5 No Cleavage; Color: metallic silver or earthy red Mineral Identity: 3. Nonmetallic Luster; Hardness: 2.5-3; Cleavage: Thin sheet; Color: black to dark brown; Uses: Construction material Mineral Identity: 4. Nonmetallic Luster; Hardness: 2.5; Cleavage: cubic; Color: colorless to white; Uses: Food additive, Melts ice Mineral Identity:
Applications and Investigations in Earth Science (9th Edition)
9th Edition
ISBN:9780134746241
Author:Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Chapter1: The Study Of Minerals
Section: Chapter Questions
Problem 1LR
Related questions
Question
100%
Hi I need help with my earth science assignment.
*Properties of Common Minerals table help with assignment I really appreciate it.

Transcribed Image Text:control
Adjunct Instructor, Graphic Design
RO005635 | Posting Date: 10/06/2021 | Lake Worth Campus
Adjunct Instructor, Paralegal
R0007510 | Posting Date: 10/01/2021 | Palm Beach Gard-
pus
NAME:
ESC - ASSIGNMENT #2 (CHAPTER 2 PART TWO): MINERAL IDENTIFICATION
DIRECTIONS: For each of the following unidentified minerals, you are provided with a
photo and some physical properties. Refer to the table of "Properties of Common Minerals"
(next page) to determine the identity of each mineral described.
Each correct answer is worth 2 points. DUE: January 19th
1. Nonmetallic Luster; Hardness: 3; Uses: Cement, Lime
Color: colorless to white; Other: Reacts (bubbles) with acid
Mineral Identity:
2. Luster: Either metallic or earthy; Hardness: 5.5 to 6.5,
but the earthy variety can be as soft as 1.0-1.5
No Cleavage; Color: metallic silver or earthy red
Mineral Identity:
3. Nonmetallic Luster; Hardness: 2.5-3;
Cleavage: Thin sheet; Color: black to dark brown;
Uses: Construction material
Mineral Identity:
4. Nonmetallic Luster; Hardness: 2.5; Cleavage: cubic;
Color: colorless to white; Uses: Food additive, Melts ice
Mineral Identity:
5. Metallic Luster; Hardness: 2.5; Cleavage: cubic;
Color: metallic silver; Streak: gray-black;
Uses: Lead ore, batteries
Mineral Identity:
delete
return
shift
Transcribed Image Text:K
CO
Nonmetallic luster
HARD-
LUSTER NESS
1-2
Either
7
9
Metallic luster
6
6
6.5
5.5
5-6
6.5-7.5
4
N↑
3.5
3
5.5-6.5
G
5.5-6.5
2.5
2.5-3
2-2.5
6.5
2
2.5
or 1
2
1
S
९
✓
९
✓
S
*Chemical symbols:
R0006520 | Posting Date: 10/28/2021
✓
Adjunct Instructor, Graphic Design
R0005635 | Posting Date: 10/06/2021 | Lake Worth Cam
✓
Adjunct Instructor, Paralegal
R0007510 | Posting Date: 10/01/2021 | Palm Beach Gardens Campus
V
V
✓
✓
COMMON
COLORS
✓= dominant form of breakage
silver to
gray
metallic
silver
black to
silver
metallic silver or
earthy red
brassy
yellow
while to
green
yellow to
amber
white to
pink or gray
colorless to
yellow
colorless to
white
black to
dark brown
colorless
or variable
colorless
or variable
dark red
to green
colorless or
variable
white to
gray
black to
dark green
black to
dark green
Al = aluminum
C = carbon
Ca = calcium
white to
pink
green to
gray or brown
colorless or
variable
Properties of Common Minerals
DISTINGUISHING
CHARACTERISTICS
black streak,
greasy feel
gray-black streak, cubic cleavage,
density=7.6 g/cm³
black streak,
magnetic
green-black streak,
(fool's gold)
red-brown streak
greasy feel
white-yellow streak
easily scratched
by fingernail
flexible in
thin sheets
cubic cleavage,
salty taste
flexible in
thin sheets
bubbles with acid,
rhombohedral cleavage
bubbles with acid
when powdered
cleaves in
4 directions
cleaves in
2 directions at 90°
cleaves at
56° and 124°
cleaves in
2 directions at 90°
CI= chlorine
F = fluorine
Fe = iron
cleaves in 2 directions,
striations visible
commonly light green
and granular
glassy luster, may form
hexagonal crystals
often seen as red glassy grains
in NYS metamorphic rocks
USE(S)
pencil lead,
lubricants
ore of lead,
batteries
ore of iron,
steel
ore of
sulfur
ore of iron,
jewelry
ceramics,
paper
sulfuric acid
plaster of paris,
drywall
paint, roofing
food additive,
melts ice
construction
materials
cement,
lime
building
stones
H = hydrogen
K = potassium
Mg = magnesium
hydrofluoric
acid
mineral collections,
jewelry
mineral collections,
jewelry
ceramics,
glass
ceramics,
glass
furnace bricks,
jewelry
glass, jewelry,
electronics
jewelry (NYS gem),
abrasives
COMPOSITION"
C
PbS
Fe304
FeS₂
Fe₂O3
Mg3Si4010(OH)2
Na = sodium
O = oxygen
Pb= lead
S
CaSO4-2H₂O
KAI3S3010(OH)2
NaCl
K(Mg,Fe)3
AISI3010(OH)2
CaCO3
CaMg(CO3)2
CaF2
(Ca,Na) (Mg,Fe,Al)
(Si,Al)₂06
CaNa(Mg,Fe)4 (Al,Fe,TI)3
Si6022(0,OH)2
KAISI308
(Na,Ca)AlSi308
MINERAL NAME
(Fe, Mg)2SO4
SIO₂
Fe3Al2S13012
S = sulfur
Si= silicon
Ti = titanium
Graphite
Galena
Magnetite
Pyrite
Hematite
Talc
Sulfur
Selenite gypsum
Muscovite mica
Halite
Biotite mica
Calcite
Dolomite
Fluorite
Pyroxene
(commonly augite)
Amphibole
(commonly hornblende)
Potassium feldspar
(commonly orthoclase)
Plagioclase feldspar
Olivine
Quartz
Garnet
B
delet
re
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Applications and Investigations in Earth Science …
Earth Science
ISBN:
9780134746241
Author:
Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:
PEARSON

Exercises for Weather & Climate (9th Edition)
Earth Science
ISBN:
9780134041360
Author:
Greg Carbone
Publisher:
PEARSON

Environmental Science
Earth Science
ISBN:
9781260153125
Author:
William P Cunningham Prof., Mary Ann Cunningham Professor
Publisher:
McGraw-Hill Education

Applications and Investigations in Earth Science …
Earth Science
ISBN:
9780134746241
Author:
Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:
PEARSON

Exercises for Weather & Climate (9th Edition)
Earth Science
ISBN:
9780134041360
Author:
Greg Carbone
Publisher:
PEARSON

Environmental Science
Earth Science
ISBN:
9781260153125
Author:
William P Cunningham Prof., Mary Ann Cunningham Professor
Publisher:
McGraw-Hill Education

Earth Science (15th Edition)
Earth Science
ISBN:
9780134543536
Author:
Edward J. Tarbuck, Frederick K. Lutgens, Dennis G. Tasa
Publisher:
PEARSON

Environmental Science (MindTap Course List)
Earth Science
ISBN:
9781337569613
Author:
G. Tyler Miller, Scott Spoolman
Publisher:
Cengage Learning

Physical Geology
Earth Science
ISBN:
9781259916823
Author:
Plummer, Charles C., CARLSON, Diane H., Hammersley, Lisa
Publisher:
Mcgraw-hill Education,