Naproxen, sold under the brand name Aleve among others, is a nonsteroidal anti- inflammatory drug (NSAID) used to treat pain (e.g. menstrual pains), inflammatory diseases such as rheumatoid arthritis, and fever. It is taken orally. The most potent enantiomer (the (S)-enantiomer) is produced by an asymmetric catalytic hydrogenation using Noyori's BINAP- ligand and ruthenium: CH3 COOH H2. [Ru(OAc),BINAP] coOOH MeO Meo (S)-Naproxen, 97% ee PPh PPH2 (R)-BINAP (i): After the reaction has completed analysis shows that the reaction mixture contains 392 g of (S)-naproxen (Mw 230,26 g/mol). How much of the (R)-enantiomer is expected to have been formed when the enantiomeric excess is 97% for the reaction? (ii): Explain briefly why the %enantiomeric excess can be regarded as a key green metric.
Naproxen, sold under the brand name Aleve among others, is a nonsteroidal anti- inflammatory drug (NSAID) used to treat pain (e.g. menstrual pains), inflammatory diseases such as rheumatoid arthritis, and fever. It is taken orally. The most potent enantiomer (the (S)-enantiomer) is produced by an asymmetric catalytic hydrogenation using Noyori's BINAP- ligand and ruthenium: CH3 COOH H2. [Ru(OAc),BINAP] coOOH MeO Meo (S)-Naproxen, 97% ee PPh PPH2 (R)-BINAP (i): After the reaction has completed analysis shows that the reaction mixture contains 392 g of (S)-naproxen (Mw 230,26 g/mol). How much of the (R)-enantiomer is expected to have been formed when the enantiomeric excess is 97% for the reaction? (ii): Explain briefly why the %enantiomeric excess can be regarded as a key green metric.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter22: Reactions Of Benzene And Its Derivatives
Section: Chapter Questions
Problem 22.59P
Related questions
Question
4
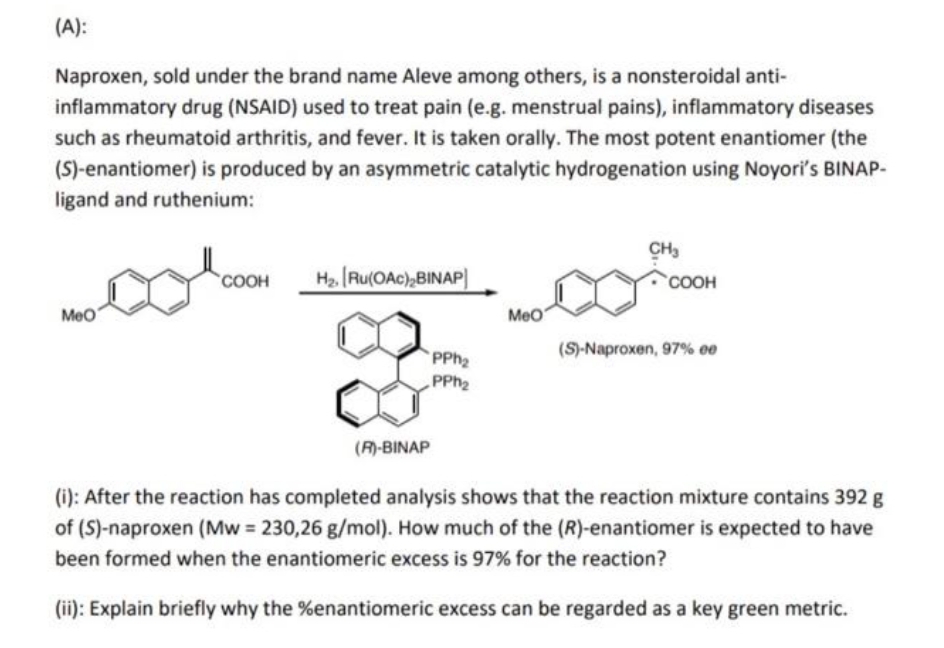
Transcribed Image Text:(A):
Naproxen, sold under the brand name Aleve among others, is a nonsteroidal anti-
inflammatory drug (NSAID) used to treat pain (e.g. menstrual pains), inflammatory diseases
such as rheumatoid arthritis, and fever. It is taken orally. The most potent enantiomer (the
(S)-enantiomer) is produced by an asymmetric catalytic hydrogenation using Noyori's BINAP-
ligand and ruthenium:
CH3
Ha Ru(OAc),BINAP)
COOH
COOH
Meo
Meo
PPH2
(S)-Naproxen, 97% 00
PPH2
(R)-BINAP
(i): After the reaction has completed analysis shows that the reaction mixture contains 392 g
of (S)-naproxen (Mw = 230,26 g/mol). How much of the (R)-enantiomer is expected to have
been formed when the enantiomeric excess is 97% for the reaction?
(ii): Explain briefly why the %enantiomeric excess can be regarded as a key green metric.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
