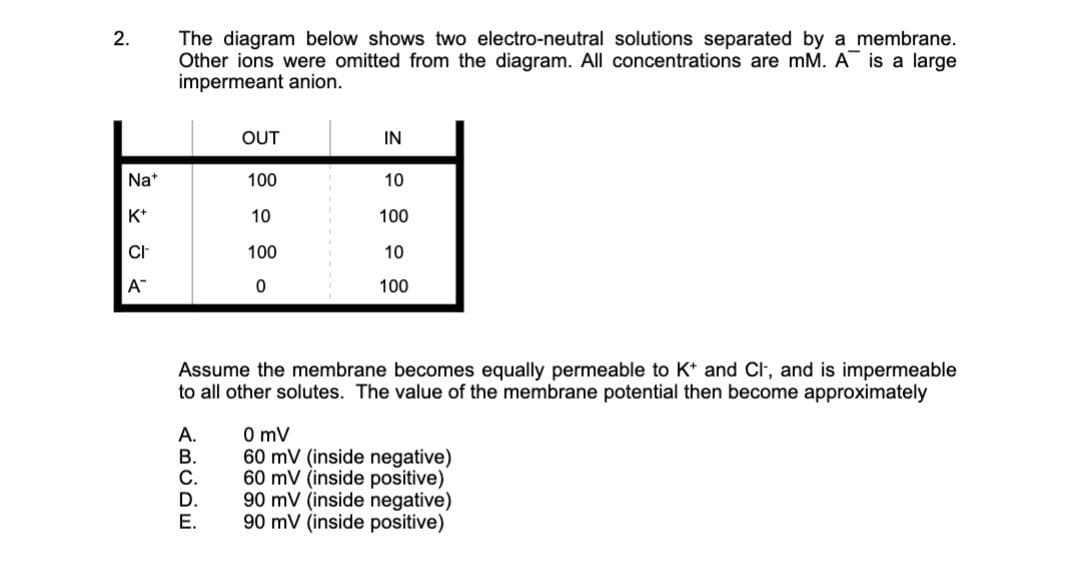
Nat K+ CI™ A™ ABCDE Assume the membrane becomes equally permeable to K+ and Cl, and is impermeable to all other solutes. The value of the membrane potential then become approximately A. 100 10 100 0 B. C. D. E. 10 100 10 100 0 mV 60 mV (inside negative) 60 mV (inside positive) 90 mV (inside negative) 90 mV (inside positive)
Nat K+ CI™ A™ ABCDE Assume the membrane becomes equally permeable to K+ and Cl, and is impermeable to all other solutes. The value of the membrane potential then become approximately A. 100 10 100 0 B. C. D. E. 10 100 10 100 0 mV 60 mV (inside negative) 60 mV (inside positive) 90 mV (inside negative) 90 mV (inside positive)
Human Physiology: From Cells to Systems (MindTap Course List)
9th Edition
ISBN:9781285866932
Author:Lauralee Sherwood
Publisher:Lauralee Sherwood
Chapter3: The Plasma Membrane And Membrane Potential
Section: Chapter Questions
Problem 2TAHL: Assume that a membrane permeable to Na+ but not to Cl- separates two solutions. The concentration of...
Related questions
Question
Transcribed Image Text:2.
Nat
K+
CI
A™
The diagram below shows two electro-neutral solutions separated by a membrane.
Other ions were omitted from the diagram. All concentrations are mM. A is a large
impermeant anion.
ABCDE
A.
B.
C.
D.
OUT
Assume the membrane becomes equally permeable to K+ and Cl, and is impermeable
to all other solutes. The value of the membrane potential then become approximately
E.
100
10
100
0
IN
10
100
10
100
0 mV
60 mV (inside negative)
60 mV (inside positive)
90 mV (inside negative)
90 mV (inside positive)

Transcribed Image Text:6.
A solution of CaCl2 (MW=110 g/mole) was prepared in water at 1.1% (w/w)
concentration. The solution
A.
B.
C.
D.
E.
has a concentration of Ca²+ close to that in normal blood plasma.
has a concentration of 300 mm.
is hypertonic relative to normal blood plasma.
is hypotonic relative to normal blood plasma.
is isotonic relative to normal blood plasma.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning

Concepts of Biology
Biology
ISBN:
9781938168116
Author:
Samantha Fowler, Rebecca Roush, James Wise
Publisher:
OpenStax College

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Human Physiology: From Cells to Systems (MindTap …
Biology
ISBN:
9781285866932
Author:
Lauralee Sherwood
Publisher:
Cengage Learning

Concepts of Biology
Biology
ISBN:
9781938168116
Author:
Samantha Fowler, Rebecca Roush, James Wise
Publisher:
OpenStax College

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Biology: The Unity and Diversity of Life (MindTap…
Biology
ISBN:
9781337408332
Author:
Cecie Starr, Ralph Taggart, Christine Evers, Lisa Starr
Publisher:
Cengage Learning

Anatomy & Physiology
Biology
ISBN:
9781938168130
Author:
Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark Womble
Publisher:
OpenStax College

Cardiopulmonary Anatomy & Physiology
Biology
ISBN:
9781337794909
Author:
Des Jardins, Terry.
Publisher:
Cengage Learning,