Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
i need help writing all of the
detailed methods: in
Preparation of Cyclohexanone by Hypochlorite Oxidation
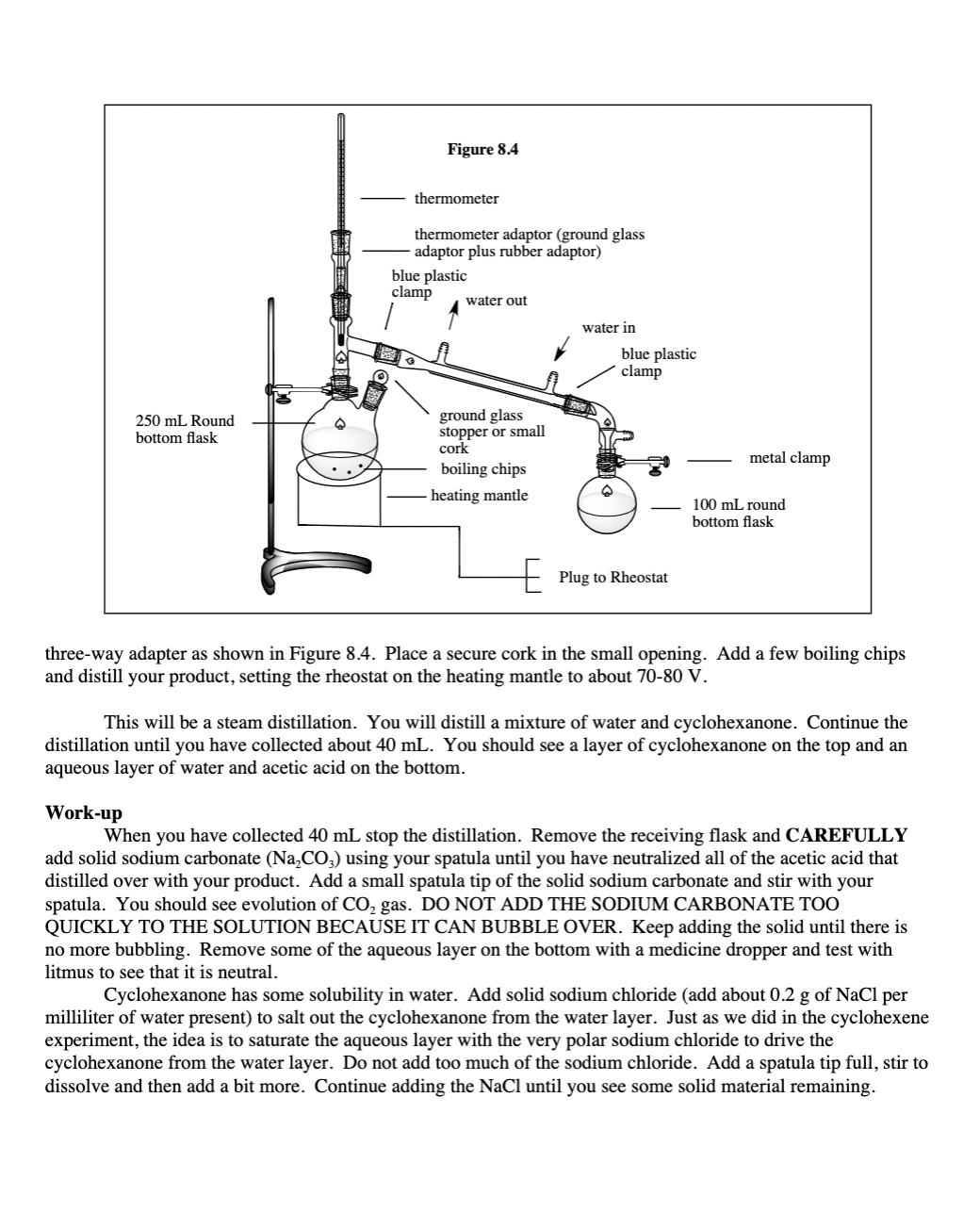
Transcribed Image Text:Figure 8.4
thermometer
thermometer adaptor (ground glass
adaptor plus rubber adaptor)
blue plastic
clamp
water out
water in
blue plastic
clamp
250 mL Round
bottom flask
ground glass
stopper or small
cork
metal clamp
boiling chips
heating mantle
100 mL round
bottom flask
+ Plug to Rheostat
three-way adapter as shown in Figure 8.4. Place a secure cork in the small opening. Add a few boiling chips
and distill your product, setting the rheostat on the heating mantle to about 70-80 V.
This will be a steam distillation. You will distill a mixture of water and cyclohexanone. Continue the
distillation until you have collected about 40 mL. You should see a layer of cyclohexanone on the top and an
aqueous layer of water and acetic acid on the bottom.
Work-up
When you have collected 40 mL stop the distillation. Remove the receiving flask and CAREFULLY
add solid sodium carbonate (Na,CO,) using your spatula until you have neutralized all of the acetic acid that
distilled over with your product. Add a small spatula tip of the solid sodium carbonate and stir with your
spatula. You should see evolution of CO, gas. DO NOT ADD THE SODIUM CARBONATE TOO
QUICKLY TO THE SOLUTION BECAUSE IT CAN BUBBLE OVER. Keep adding the solid until there is
no more bubbling. Remove some of the aqueous layer on the bottom with a medicine dropper and test with
litmus to see that it is neutral.
Cyclohexanone has some solubility in water. Add solid sodium chloride (add about 0.2 g of NaCl per
milliliter of water present) to salt out the cyclohexanone from the water layer. Just as we did in the cyclohexene
experiment, the idea is to saturate the aqueous layer with the very polar sodium chloride to drive the
cyclohexanone from the water layer. Do not add too much of the sodium chloride. Add a spatula tip full, stir to
dissolve and then add a bit more. Continue adding the NaCl until you see some solid material remaining.
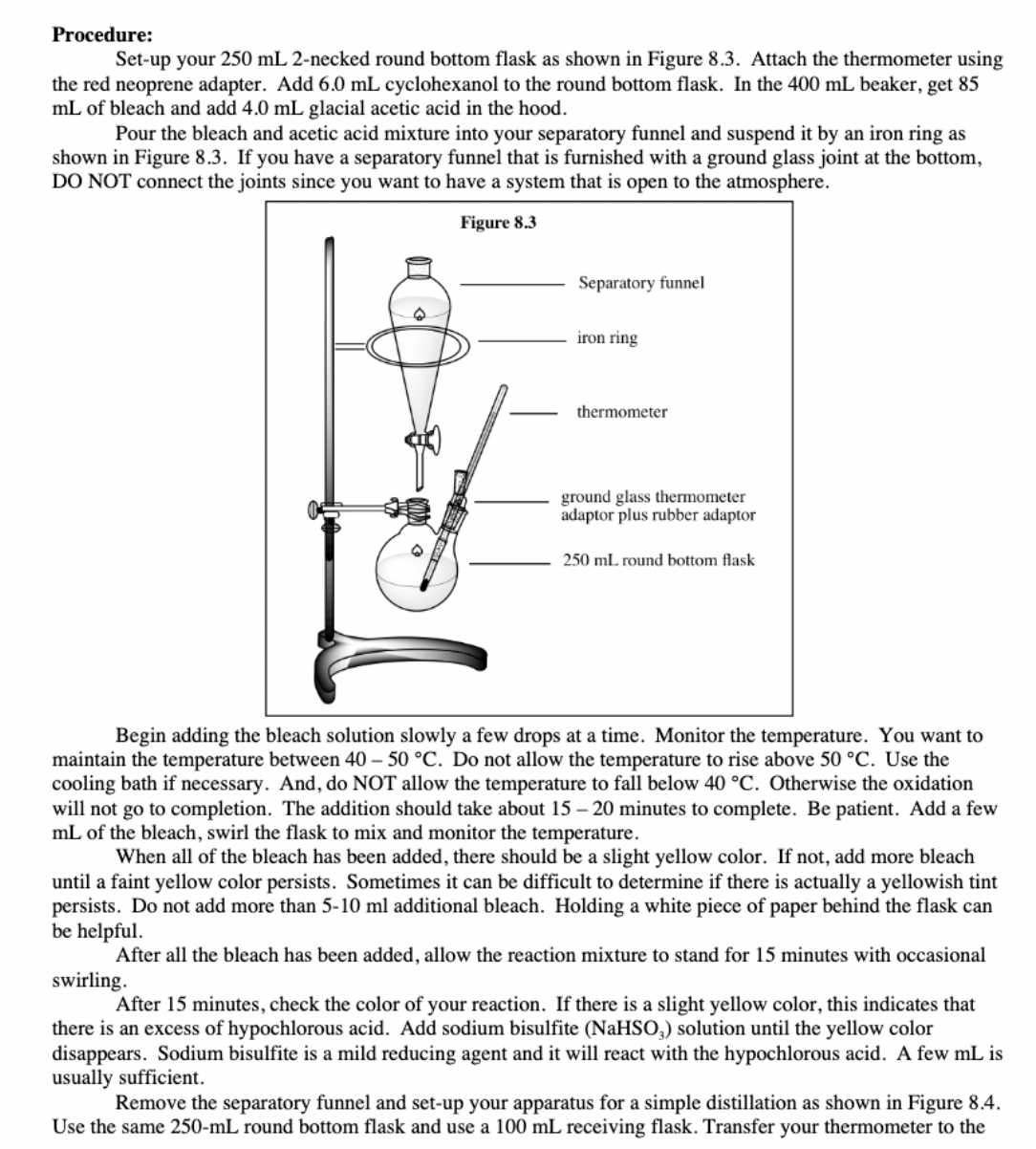
Transcribed Image Text:Procedure:
Set-up your 250 mL 2-necked round bottom flask as shown in Figure 8.3. Attach the thermometer using
the red neoprene adapter. Add 6.0 mL cyclohexanol to the round bottom flask. In the 400 mL beaker, get 85
mL of bleach and add 4.0 mL glacial acetic acid in the hood.
Pour the bleach and acetic acid mixture into your separatory funnel and suspend it by an iron ring as
shown in Figure 8.3. If you have a separatory funnel that is furnished with a ground glass joint at the bottom,
DO NOT connect the joints since you want to have a system that is open to the atmosphere.
Figure 8.3
Separatory funnel
iron ring
thermometer
ground glass thermometer
adaptor plus rubber adaptor
250 mL round bottom flask
Begin adding the bleach solution slowly a few drops at a time. Monitor the temperature. You want to
maintain the temperature between 40 – 50 °C. Do not allow the temperature to rise above 50 °C. Use the
cooling bath if necessary. And, do NOT allow the temperature to fall below 40 °C. Otherwise the oxidation
will not go to completion. The addition should take about 15 – 20 minutes to complete. Be patient. Add a few
mL of the bleach, swirl the flask to mix and monitor the temperature.
When all of the bleach has been added, there should be a slight yellow color. If not, add more bleach
until a faint yellow color persists. Sometimes it can be difficult to determine if there is actually a yellowish tint
persists. Do not add more than 5-10 ml additional bleach. Holding a white piece of paper behind the flask can
be helpful.
After all the bleach has been added, allow the reaction mixture to stand for 15 minutes with occasional
swirling.
After 15 minutes, check the color of your reaction. If there is a slight yellow color, this indicates that
there is an excess of hypochlorous acid. Add sodium bisulfite (NaHSO,) solution until the yellow color
disappears. Sodium bisulfite is a mild reducing agent and it will react with the hypochlorous acid. A few mL is
usually sufficient.
Remove the separatory funnel and set-up your apparatus for a simple distillation as shown in Figure 8.4.
Use the same 250-mL round bottom flask and use a 100 mL receiving flask. Transfer your thermometer to the
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY