Netflix Flipgrid | B0I ay General. (References)] (Review Topics) Use the References to access important values if needed for this question. Salt bridge A concentration cell similar to the one shown is composed of two Al electrodes and solutions of different Al* concentrations. The left compartrment contains 0.793 M AP, and the right compartment contains 0.614 M A*. Calculate the cell potential for this reaction at 298 K. volts In this aluminum concentration cell, the reaction would proceed sntaneously
Netflix Flipgrid | B0I ay General. (References)] (Review Topics) Use the References to access important values if needed for this question. Salt bridge A concentration cell similar to the one shown is composed of two Al electrodes and solutions of different Al* concentrations. The left compartrment contains 0.793 M AP, and the right compartment contains 0.614 M A*. Calculate the cell potential for this reaction at 298 K. volts In this aluminum concentration cell, the reaction would proceed sntaneously
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 105QAP: Consider a voltaic cell in which the following reaction occurs. Zn(s)+Sn2+(aq)Zn2+(aq)+Sn(s) (a)...
Related questions
Question
Transcribed Image Text:Favorites
Netflix
GRLContent
Content
Netflix
Flipgrid | 50ffc..
ay General.
(References)
(Review Topics)
Use the References to access important values if needed for this question.
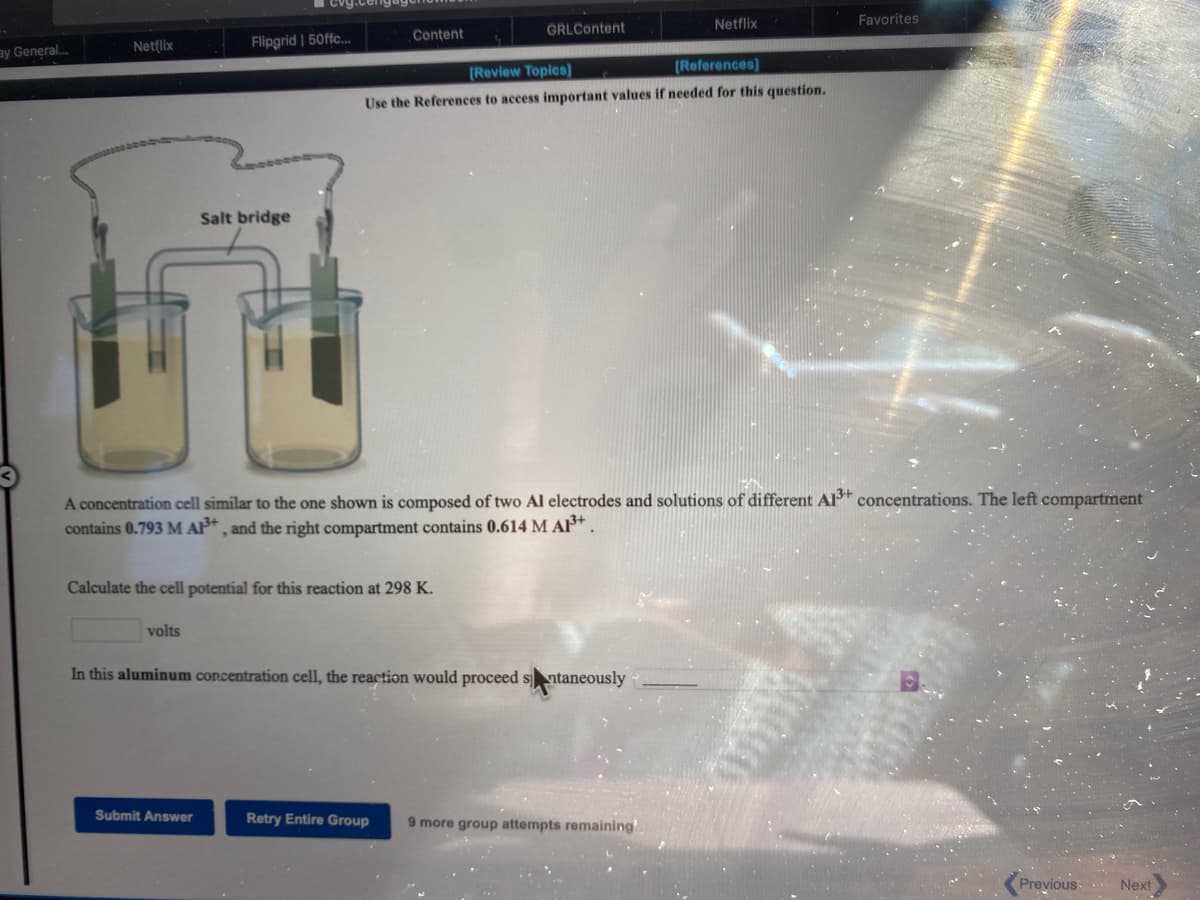
Salt bridge
A concentration cell similar to the one shown is composed of two Al electrodes and solutions of different Al* concentrations. The left compartrment
contains 0.793 M AP* , and the right compartment contains 0.614 M AP*
Calculate the cell potential for this reaction at 298 K.
volts
In this aluminum concentration cell, the reaction would proceed sntaneously
Submit Answer
Retry Entire Group
9 more group attempts remaining
Previous
Next
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning