Nitric acid and nitrogen monoxide react to form nitrogen dioxide and water, like this: 2 HNO3(aq)+NO(g)→3 NO,(9)+H,O(1) At a certain temperature, a chemist finds that a 4.0 L reaction vessel containing a mixture of nitric acid, nitrogen monoxide, nitrogen dioxide, and water at equilibrium has the following composition: |compound amount HNO3 5.2 g NO 19.8 g NO, 7.9 g H,0 134.3 g Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K. - 0
Nitric acid and nitrogen monoxide react to form nitrogen dioxide and water, like this: 2 HNO3(aq)+NO(g)→3 NO,(9)+H,O(1) At a certain temperature, a chemist finds that a 4.0 L reaction vessel containing a mixture of nitric acid, nitrogen monoxide, nitrogen dioxide, and water at equilibrium has the following composition: |compound amount HNO3 5.2 g NO 19.8 g NO, 7.9 g H,0 134.3 g Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. K. - 0
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.34PAE: 1’he reaction in Exercise 12.33 was repeated. This time, the reaction began when only NO was...
Related questions
Question
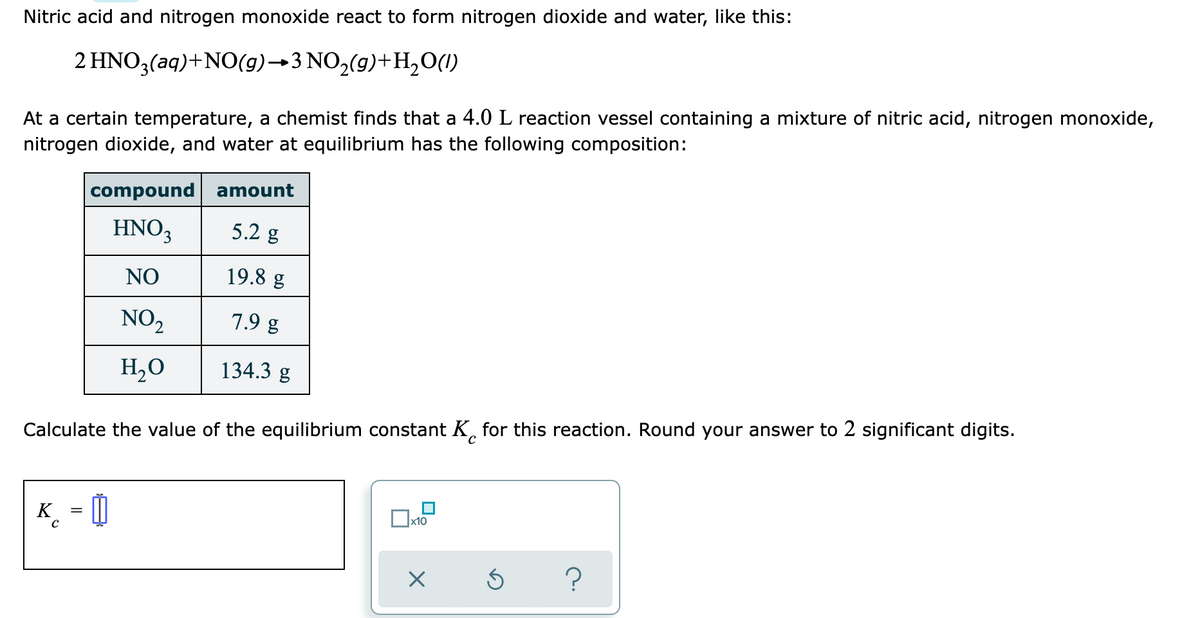
Transcribed Image Text:Nitric acid and nitrogen monoxide react to form nitrogen dioxide and water, like this:
2 HNO3(aq)+NO(9)→3 NO2(g)+H,0(1)
At a certain temperature, a chemist finds that a 4.0 L reaction vessel containing a mixture of nitric acid, nitrogen monoxide,
nitrogen dioxide, and water at equilibrium has the following composition:
compound amount
HNO3
5.2 g
NO
19.8 g
NO,
7.9 g
H,O
134.3 g
Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits.
°C
K_ = 0
x10
C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning
